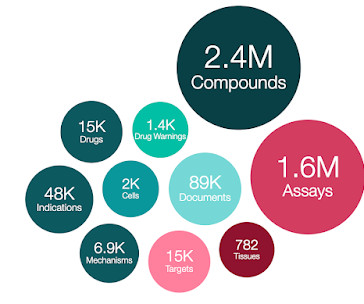
ChEMBL 34 is out!
The ChEMBL-og
APRIL 16, 2024
71 out of the 882 newly added EMA drugs are only authorised by EMA, rather than from other regulatory bodies e.g. FDA. Progress has been made towards standardising drug and clinical candidate pref_names (in MOLECULE_DICTIONARY.PREF_NAME) whereby an approved drug name (FDA /EMA) is assigned in the first instance, if available.







Let's personalize your content