NOW AVAILABLE: The 2024 Economic Report on U.S. Pharmacies and Pharmacy Benefit Managers
Drug Channels
MARCH 19, 2024
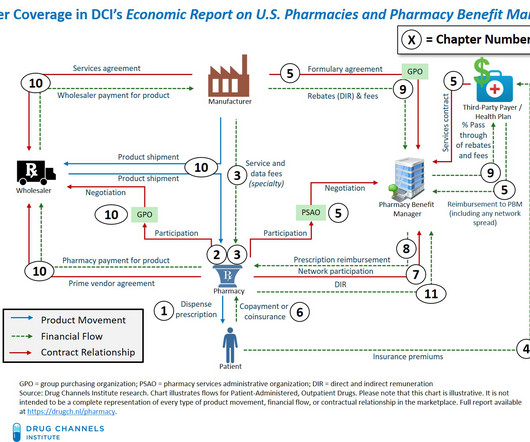
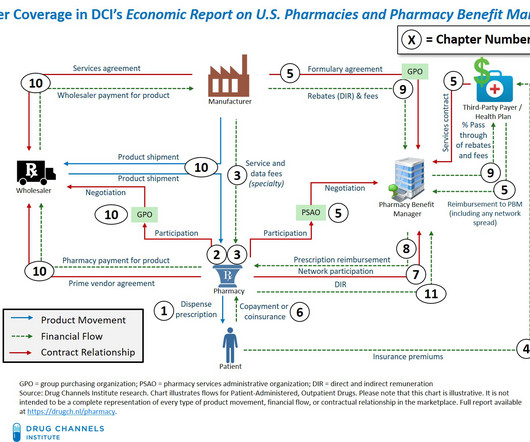
I am pleased to announce our new 2024 Economic Report on U.S. Pharmacies and Pharmacy Benefit Managers , available for purchase and immediate download. The 2024 Economic Report on U.S. This 2024 edition includes substantial new material—outlined on page ix of the report overview. All rights reserved.






















Let's personalize your content