Biosimilars are gaining ground. The IRA could push them further next year.
BioPharma Drive: Drug Pricing
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.

Drugs.com
FEBRUARY 19, 2025
19, 2025 -- The U.S. Food and Drug Administration has approved Merilog (insulin-aspart-szjj) as a biosimilar to Novolog (insulin aspart) for adults and pediatric patients with diabetes mellitus. WEDNESDAY, Feb. Merilog is a rapid-acting human.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drugs.com
FEBRUARY 19, 2025
19, 2025 -- The U.S. Food and Drug Administration has approved Merilog (insulin-aspart-szjj) as a biosimilar to Novolog (insulin aspart) for adults and pediatric patients with diabetes mellitus.Merilog is a rapid-acting human. WEDNESDAY, Feb.
Drug Patent Watch
DECEMBER 10, 2024
Biosimilars have been transforming the pharmaceutical landscape by offering cost-effective alternatives to biologic drugs. As patents for these biologics expire, the market for biosimilars is expanding rapidly, with significant implications for manufacturing technologies.
Drug Channels
SEPTEMBER 4, 2024
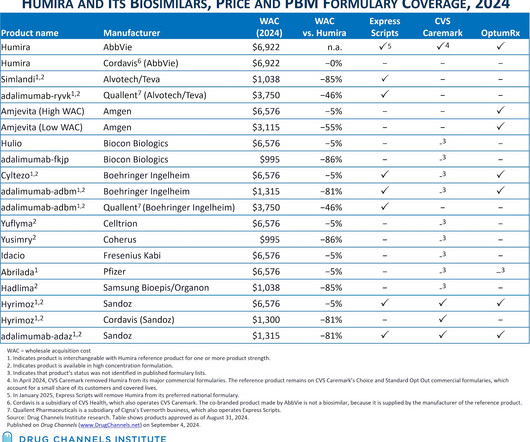
The Humira biosimilar market just took another step forward—but remains far from its ideal state. Last week, Cigna’s Express Scripts announced that it that will follow CVS Health’s CVS Caremark business and remove Humira from its largest commercial formulary in favor of multiple biosimilars.

FDA Law Blog: Biosimilars
APRIL 7, 2025
Lets take a look at these letters: Back in January 2025, CVM issued a Warning Letter to animal drug sponsor Elanco Animal Health. We note that CVM has issued even more enforcement actions in 2025 than on the human side: OPDP has issued 2 Untitled Letters so far in 2025 compared with CVMs 3 enforcement actions.

FDA Law Blog: Biosimilars
SEPTEMBER 4, 2024
HP&M”) is proud to announce that 16 of the Firm’s attorneys have been selected to the 2025 edition of The Best Lawyers in America®. Hyman has been rightfully chosen as a 2025 “Lawyer of the Year.” Hyman, Phelps & McNamara, P.C. (“HP&M”) Founding Director Paul M. Additionally, Kalie E. Richardson, James E.

Agency IQ
JULY 26, 2024
FDA’s new guidance on postapproval manufacturing changes for biosimilars focuses on current practice, new dosage forms Meeting a biosimilar user fee commitment, the FDA is expanding on its recommendations for biosimilar and interchangeable product applicants asking the FDA for post-approval manufacturing changes.

FDA Law Blog: Biosimilars
FEBRUARY 26, 2025
A 2025 study reported by Radical Compliance found that while senior management often believes in the robustness of their ethical culture, employees at other levels do not. Department of Justices Consumer Protection Branch.

FDA Law Blog: Biosimilars
APRIL 7, 2025
Come together with leaders from the pharmaceutical, biotechnology, and diagnostic industries to unpack huge changes at the American Conference Institutes 23rd Advanced Summit on Life Sciences Patents , which is scheduled to take place from May 19-20, 2025 at the New York Bar Association in New York, NY.

FDA Law Blog: Biosimilars
MARCH 18, 2025
On March 13, 2025, the Federal Circuit ventured into the world of reissued patents and PTE. ( And just last week, a new question was answered by the Federal Circuit: how is a patent term extension calculated for a reissued patent? Is it based on the issue date of the original patent or the reissued patent? In Merck Sharp & Dohme Corp.

Drug Channels
JUNE 25, 2024
Attendance will be limited, so click here to request an invite for our March 2025 event. Happy 248th birthday, America! Read more » © 2006-2024 HMP Omnimedia, LLC d/b/a Drug Channels Institute , an HMP Global Company. All rights reserved. This Feed is for personal non-commercial use only.

FDA Law Blog: Biosimilars
SEPTEMBER 11, 2024
Last week’s guidance is featured on FDA’s new nitrosamines webpage and recommends that manufacturers implement revisions to control measures by August 1, 2025, noting that manufacturers and sponsors of approved products were expected to complete evaluations for small molecule nitrosamines last year.

FDA Law Blog: Biosimilars
FEBRUARY 27, 2025
The American Conference Institutes popular FDA Boot Camp now in its 43rd iteration is scheduled to take place from March 19-20, 2025, at the NY Bar Association in New York, NY. The conference is billed as the premier event to provide folks with a roadmap to navigate the difficult terrain of FDA regulatory law. And it is exactly that!

FDA Law Blog: Drug Discovery
MARCH 3, 2025
The American Conference Institutes 3rd Annual Forum on Advanced Therapeutics is scheduled to take place from March 19-20, 2025, at the Seaport Hotel in Boston, MA.

FDA Law Blog: Biosimilars
DECEMBER 3, 2023
On November 17th, CMS issued its final guidance on the Discount Program in which it responded to public comments and provided updated guidance for the Discount Program for 2025 and 2026. Manufacturers must sign the agreement by March 1, 2024, to participate in the 2025 plan year. Final Guidance at 2.

The Pharma Data
MAY 18, 2021
Sterile API production is planned to transfer from Kundl to the new facility at Palafolls in 2025. Neither can there be any guarantee that, if approved, such generic or biosimilar products will be approved for all indications included in the reference product’s label. About Sandoz.

The Pharma Data
JULY 29, 2021
Looking forward, we remain highly confident in our ability to achieve at least a 6% compound annual growth rate through 2025 and intend to build upon our recent successes by continuing to follow the science, trust in our people and remain focused on delivering breakthroughs for the patients we serve.”. Revenues $45.0 to $2.60).

FDA Law Blog: Biosimilars
DECEMBER 12, 2024
Last weeks prehearing conference set the parameters for the hearing on the merits, scheduled to begin January 21, 2025. The purpose of the public hearing is to receive factual evidence and expert opinion testimony on whether marijuana should be rescheduled to schedule III. Prehearing Ruling (Dec. 4, 2024), at 1.

FDA Law Blog: Biosimilars
SEPTEMBER 25, 2024
Throughout his illustrious career, Al has been engaged with Hatch-Waxman, even authoring one of the earliest papers providing an account of the law: “ Special Patent Provisions for Pharmaceuticals: Have They Outlived Their Usefulness? ” In his new book, “Breaking the Medicine Monopolies: Reflections of a Generic Drug Pioneer,” which will be released (..)

FDA Law Blog: Biosimilars
MARCH 21, 2024
MoCRA requires that FDA issue a report on PFAS in cosmetics by the end of 2025, but one takeaway from these discussions was that several states laws create a patchwork of relevant laws, further complicated by a plaintiffs’ bar that is aggressively pursuing class action lawsuits.

FDA Law Blog: Biosimilars
JUNE 14, 2023
In FY 2025, continue to support products enrolled in previous fiscal years and expand to enroll up to 65 additional products in at least four OHTs (i.e., up to 125 total products enrolled through FY 2025). up to 60 total products enrolled through FY 2024).

FDA Law Blog: Biosimilars
JULY 7, 2024
The first phaseout milestone is less than a year away; by May 6, 2025 most laboratories will need to demonstrate compliance with Medical Device Reporting (21 C.F.R. § 803), Reporting of Corrections and Removals (21 C.F.R. § 806) and Complaint Files (21 C.F.R.

FDA Law Blog: Biosimilars
JULY 6, 2023
We remain cautiously optimistic of the timeline given the extensive and competing priorities CDRH has in front of them, including: implementing provisions required under the Food and Drug Omnibus Reform Act (FDORA) (which we summarized and analyzed here ), meeting performance goals set forth in MDUFA V , working on their 2022 – 2025 strategic priorities (..)

FDA Law Blog: Biosimilars
SEPTEMBER 22, 2024
Stage 1 is scheduled to take effect on May 6, 2025. See our prior blog post summarizing the different phaseout stages and categories of enforcement discretion. A: According to the Agency, the lab would be considered a manufacturer of the new, modified test and must follow applicable regulatory requirements, including MDR requirements.

FDA Law Blog: Biosimilars
OCTOBER 12, 2023
Priority is given to Suitability Petitions that could mitigate or resolve a drug shortage; address a public health emergency declared by HHS; mitigate waste by way of new strengths for parenteral products; or subject to special review under the President’s Emergency Plan for Aids Relief.

Agency IQ
AUGUST 2, 2024
All clinical trials still ongoing must transition to the Clinical Trials Regulation by January 31, 2025 to ensure continuity. legislation The list below includes legislation proposed by the European Commission that is yet-to-be-published.

FDA Law Blog: Biosimilars
OCTOBER 24, 2023
The draft guidance indicates that this timetable will be announced by September 30, 2025. Timeline FDA notes that when this draft guidance is finalized, it will specify the corresponding timetable(s) for the implementation of De Novo electronic submissions.

Agency IQ
JULY 8, 2024
Date What’s Happening Explanation Source June 29 GGP Report FDA expected to finalize report on Good Guidance Practices AgencyIQ June 30 User Fee Payments Industry required to confirm NDA prescription drug products in Orange Book for purposes of PDUFA payments in FY 2025 FDA July 1 Anniversary Anniversary of the signing of the Public Health Service (..)

Agency IQ
MAY 3, 2024
Positive *Biosimilar **Generic Notable public consultation periods and calls for evidence Below are various deadlines regarding European policy, such as public registers, drafts, reflection papers, concept papers, and guidance documents expected to close into Q2 2024.

Agency IQ
SEPTEMBER 1, 2023
Products eligible to qualify for the IRP include chemical and biological new or known active substances ( Regulation 50 ), generics ( Regulation 51 ), biosimilars ( Regulation 53 ) and fixed combination products ( Regulation 55 ). centralized procedure until 2025, when the Windsor Framework takes effect. IRP approvals using the E.U.

Agency IQ
MAY 31, 2024
Program Agency Deadline Survey: EU4Health Annual Work Program 2025 European Commission 6/10/2024 Survey: Real4Reg survey on real-world data Real4Reg/EMA 6/14/2024 Survey: EMA Communication perception EMA 6/21/2024 Survey: E.U. These include, but are not limited to, calls for applicants, expressions of interest and surveys.

The Pharma Data
FEBRUARY 4, 2021
Percent on an Operational Basis, Due to Biosimilar Competition; Global Skyrizi Net Revenues Were $1.590 Billion; Global Rinvoq Net Revenues Were $731 Million. percent on an operational basis, due to biosimilar competition. Humira Net Revenues Were $16.112 Billion, an Increase of 8.4 Percent on a Reported Basis, or 12.5

FDA Law Blog: Biosimilars
JUNE 7, 2023
the Medicaid Drug Rebate Agreement and the Medicare Part D Coverage Gap Discount Agreement or the agreement under the successor Part D discount program that begins in 2025), Merck characterizes that as a coercive penalty for the refusal to forfeit its First and Fifth amendment rights.

Agency IQ
MARCH 29, 2024
Title Type Deadline Guideline on clinical investigation of medicinal products in the treatment of depression [EMA] Scientific guideline 3/31/2024 Concept paper on the establishment of a Guideline on the development and manufacture of human medicinal products specifically designed for phage therapy [EMA] Concept paper 3/31/2024 Pharmeuropa 36.1

Agency IQ
JULY 8, 2024
Negative *Biosimilar **Generic Notable public consultation periods and calls for evidence Below are various deadlines regarding European policy, such as public registers, drafts, reflection papers, concept papers, and guidance documents expected to close into Q3 2024.

Agency IQ
OCTOBER 27, 2023
11/9/2023: Enters into force 11/9/2025: Date of application To contact the authors of this resource, please email Sierra Milam ( smilam@agencyiq.com ). To contact the editor of this resource, please email Chelsey McIntyre ( cmcintyre@agencyiq.com ).

FDA Law Blog: Biosimilars
JUNE 21, 2023
Background – Strategic Priority to Advance Health Equity In CDRH’s 2022-2025 Strategic Priorities , “Advance Health Equity” is listed as one of the three strategic priorities along with “Promote a Modern and Diverse Workforce” and “Enhance Organizational Agility and Resilience.”

The Pharma Data
MAY 4, 2021
Even excluding the growth provided from BNT162b2, our revenues grew 8% operationally, which aligns with our stated goal of delivering at least a 6% compound annual growth rate through 2025. CAPITAL ALLOCATION. Inlyta globally, up 34% operationally, primarily reflecting increased demand in the U.S. billion on revenues and approximately $0.09

The Pharma Data
FEBRUARY 8, 2021
price, patients switching to Toujeo ® and biosimilar glargine competition. to €189 million) reflecting recent guidelines recommending the use of low molecular weight heparins in hospitalized COVID-19 patients which more than offset biosimilar competition in several European countries. Full-year 2020 franchise sales were down 4.8%

The Pharma Data
FEBRUARY 4, 2021
price, patients switching to Toujeo ® and biosimilar glargine competition. to €189 million) reflecting recent guidelines recommending the use of low molecular weight heparins in hospitalized COVID-19 patients which more than offset biosimilar competition in several European countries. Full-year 2020 franchise sales were down 4.8%

Agency IQ
OCTOBER 27, 2023
WUSF / AgencyIQ November 1 Initial deadline for NDSRIs Under a 2023 guidance document, the FDA has recommended that pharmaceutical companies assess Nitrosamine Drug-Related Substance Impurities for their products by November 1, 2023, with confirmatory testing due by August 1, 2025.

Agency IQ
FEBRUARY 15, 2024
In other cases, the FDA is under no obligation to release a document at any time, but is instead developing the document on its own accord. We have tried to sort guidance documents by topic area.

FDA Law Blog: Biosimilars
MAY 23, 2024
Even if the upcoming elections result in a change in Presidential Administration or composition of the Senate, FDA’s Final Rule would be ineligible for review under the CRA, as the 60 legislative days will have elapsed by January 2025. The lab industry should be watching closely for litigation and legislative developments.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content