Biosimilars are gaining ground. The IRA could push them further next year.
BioPharma Drive: Drug Pricing
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.
Drug Patent Watch
DECEMBER 10, 2024
Biosimilars have been transforming the pharmaceutical landscape by offering cost-effective alternatives to biologic drugs. As patents for these biologics expire, the market for biosimilars is expanding rapidly, with significant implications for manufacturing technologies.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
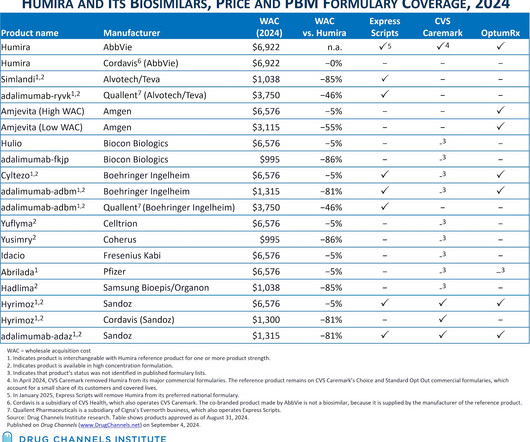
Drug Channels
SEPTEMBER 4, 2024
The Humira biosimilar market just took another step forward—but remains far from its ideal state. Last week, Cigna’s Express Scripts announced that it that will follow CVS Health’s CVS Caremark business and remove Humira from its largest commercial formulary in favor of multiple biosimilars.

FDA Law Blog: Biosimilars
FEBRUARY 26, 2025
M&A rises and falls on due diligence along with other factors like market conditions and growth opportunities, so compliance and quality are vital links in any deal chain. For one thing, the possible answers to our rhetorical questions above are not final yet and will take years to sort out.

Agency IQ
JULY 26, 2024
FDA’s new guidance on postapproval manufacturing changes for biosimilars focuses on current practice, new dosage forms Meeting a biosimilar user fee commitment, the FDA is expanding on its recommendations for biosimilar and interchangeable product applicants asking the FDA for post-approval manufacturing changes.

FDA Law Blog: Drug Discovery
MARCH 3, 2025
The American Conference Institutes 3rd Annual Forum on Advanced Therapeutics is scheduled to take place from March 19-20, 2025, at the Seaport Hotel in Boston, MA. Attend to engage with leading industry thought leaders and legal pioneers who will illuminate the path from groundbreaking research to global market success.

FDA Law Blog: Biosimilars
DECEMBER 3, 2023
On November 17th, CMS issued its final guidance on the Discount Program in which it responded to public comments and provided updated guidance for the Discount Program for 2025 and 2026. Manufacturers must sign the agreement by March 1, 2024, to participate in the 2025 plan year. Final Guidance at 2.

The Pharma Data
JULY 29, 2021
Looking forward, we remain highly confident in our ability to achieve at least a 6% compound annual growth rate through 2025 and intend to build upon our recent successes by continuing to follow the science, trust in our people and remain focused on delivering breakthroughs for the patients we serve.”. Revenues $45.0 to $2.60).

FDA Law Blog: Biosimilars
MARCH 21, 2024
MoCRA requires that FDA issue a report on PFAS in cosmetics by the end of 2025, but one takeaway from these discussions was that several states laws create a patchwork of relevant laws, further complicated by a plaintiffs’ bar that is aggressively pursuing class action lawsuits.

The Pharma Data
MAY 18, 2021
By modernizing and simplifying its manufacturing setup, Sandoz will improve its ability to consistently deliver high-quality medicines to patients, while remaining cost-competitive on the global market. Sterile API production is planned to transfer from Kundl to the new facility at Palafolls in 2025. About Sandoz.

FDA Law Blog: Biosimilars
JUNE 14, 2023
The striking phrase “valley of death,” is generally understood to refer to the tendency for innovative technologies to fail to reach market, whether due to reimbursement and/or physician or patient preference. up to 125 total products enrolled through FY 2025). The initial target for this simplification effort is the pre-market phase.

FDA Law Blog: Biosimilars
SEPTEMBER 22, 2024
The LDT-specific Product Codes are as follows: SCE : IVD offered as LDT, first marketed before May 6, 2024, and not modified beyond scope described in preamble to LDT Final Rule. Stage 1 is scheduled to take effect on May 6, 2025. SCF : LDT, unmet need within an integrated healthcare system. 21 CFR 820.20

Agency IQ
SEPTEMBER 1, 2023
s MHRA unveiled details of its new International Recognition Procedure, which will allow the MHRA to rely on marketing authorizations by reference regulators from several countries for a wide range of products, including generics and those that received expedited review. market more quickly. The procedure is available for E.U.

FDA Law Blog: Biosimilars
JULY 7, 2024
The first phaseout milestone is less than a year away; by May 6, 2025 most laboratories will need to demonstrate compliance with Medical Device Reporting (21 C.F.R. § 803), Reporting of Corrections and Removals (21 C.F.R. § 806) and Complaint Files (21 C.F.R. report certain device malfunctions, and.

Agency IQ
JULY 8, 2024
Date What’s Happening Explanation Source June 29 GGP Report FDA expected to finalize report on Good Guidance Practices AgencyIQ June 30 User Fee Payments Industry required to confirm NDA prescription drug products in Orange Book for purposes of PDUFA payments in FY 2025 FDA July 1 Anniversary Anniversary of the signing of the Public Health Service (..)

The Pharma Data
MAY 4, 2021
Even excluding the growth provided from BNT162b2, our revenues grew 8% operationally, which aligns with our stated goal of delivering at least a 6% compound annual growth rate through 2025. and four additional selling days in international markets. This guidance may be adjusted in the future as additional contracts are executed.

Agency IQ
OCTOBER 27, 2023
11/9/2023: Enters into force 11/9/2025: Date of application To contact the authors of this resource, please email Sierra Milam ( smilam@agencyiq.com ). Upcoming Webinar Hosted by Xtalks Webinar/Seminar ( OPEN) Xtalks 11/6/2023 11/6/2023 What is the evidence for high-risk medical devices in the field of cardiovascular disease and diabetes?

Agency IQ
AUGUST 2, 2024
All clinical trials still ongoing must transition to the Clinical Trials Regulation by January 31, 2025 to ensure continuity. legislation The list below includes legislation proposed by the European Commission that is yet-to-be-published.

Agency IQ
MAY 3, 2024
Positive *Biosimilar **Generic Notable public consultation periods and calls for evidence Below are various deadlines regarding European policy, such as public registers, drafts, reflection papers, concept papers, and guidance documents expected to close into Q2 2024.

Agency IQ
MAY 31, 2024
Program Agency Deadline Survey: EU4Health Annual Work Program 2025 European Commission 6/10/2024 Survey: Real4Reg survey on real-world data Real4Reg/EMA 6/14/2024 Survey: EMA Communication perception EMA 6/21/2024 Survey: E.U. and 2 mg [EMA] Product-specific bioequivalence guidance 6/30/2024 Pharmeuropa 36.2

Agency IQ
JULY 8, 2024
The outgoing Belgian Presidency further complicated discussions in the “incentives cluster” by suggesting four options that aim to ensure market access. Some “add-on” regulatory data protection may also be converted to market exclusivity. However, this was the first public debate and Hungary will take over the Presidency next week.

The Pharma Data
FEBRUARY 4, 2021
Percent on an Operational Basis, Due to Biosimilar Competition; Global Skyrizi Net Revenues Were $1.590 Billion; Global Rinvoq Net Revenues Were $731 Million. percent on an operational basis, due to biosimilar competition. Humira Net Revenues Were $16.112 Billion, an Increase of 8.4 Percent on a Reported Basis, or 12.5 Recorded a $4.7

Agency IQ
MARCH 29, 2024
Title Type Deadline Guideline on clinical investigation of medicinal products in the treatment of depression [EMA] Scientific guideline 3/31/2024 Concept paper on the establishment of a Guideline on the development and manufacture of human medicinal products specifically designed for phage therapy [EMA] Concept paper 3/31/2024 Pharmeuropa 36.1

The Pharma Data
FEBRUARY 8, 2021
to €115 million reflecting continued growth in AD in key countries and additional launches in asthma in European markets. price, patients switching to Toujeo ® and biosimilar glargine competition. driven by Rest of the World sales growth which more than offset biosimilar competition in Europe. Plavix ® sales were down 1.4%

The Pharma Data
FEBRUARY 4, 2021
to €115 million reflecting continued growth in AD in key countries and additional launches in asthma in European markets. price, patients switching to Toujeo ® and biosimilar glargine competition. driven by Rest of the World sales growth which more than offset biosimilar competition in Europe. Plavix ® sales were down 1.4%

Agency IQ
OCTOBER 27, 2023
WUSF / AgencyIQ November 1 Initial deadline for NDSRIs Under a 2023 guidance document, the FDA has recommended that pharmaceutical companies assess Nitrosamine Drug-Related Substance Impurities for their products by November 1, 2023, with confirmatory testing due by August 1, 2025. the drug and device) of the combination product.

Agency IQ
FEBRUARY 15, 2024
In other cases, the FDA is under no obligation to release a document at any time, but is instead developing the document on its own accord. We have tried to sort guidance documents by topic area. Priority A List.

Agency IQ
DECEMBER 1, 2023
If all changes are accepted, the date of application is set to be January 1, 2025. Positive *Biosimilar **Generic Notable comment periods closing in December Below are various deadlines regarding E.U. Expected Action Description of action Date Eudamed Module: Market Surveillance [E.U.]
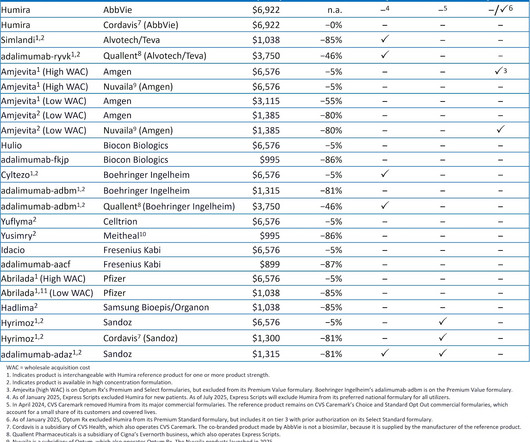
Drug Channels
JANUARY 22, 2025
For 2025, the three largest pharmacy benefit managers (PBMs)Caremark (CVS Health), Express Scripts (Cigna), and Optum Rx (United Health Group)have again each excluded hundreds of drugs from their standard formularies. In fact, nearly all marketed Humira biosimilars are excluded from the larger PBMs 2025 formularies.

Drug Patent Watch
JANUARY 13, 2025
Biosimilars have emerged as a game-changing force, promising to revolutionize patient access to life-saving biologics while simultaneously reducing healthcare costs. ”[1] The global biosimilars market is experiencing exponential growth, with projections indicating it will reach $69.4 from 2020 to 2025[1].
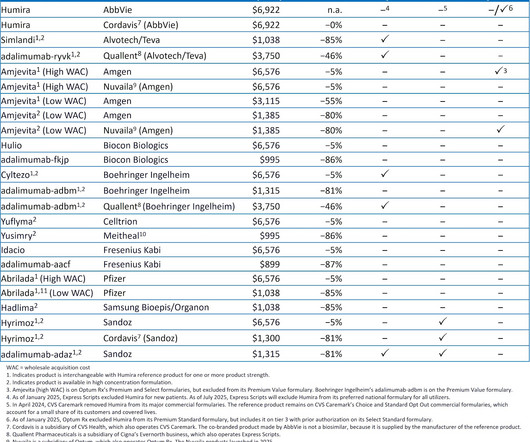
Drug Channels
APRIL 1, 2025
Click here to see the original post from January 2025. For 2025, the three largest pharmacy benefit managers (PBMs)Caremark (CVS Health), Express Scripts (Cigna), and Optum Rx (United Health Group)have again each excluded hundreds of drugs from their standard formularies. What do you think? All rights reserved.

Drug Patent Watch
MARCH 18, 2025
Exploring the Russian Pharma Industry: Key Players and Innovations As we continue to navigate the complexities of the global pharmaceutical landscape, it's essential to stay informed about emerging markets and trends. According to a recent report, the Russian pharma market is expected to reach $24.5

Drug Channels
APRIL 3, 2025
Click here to see the original post from February 2025. ICYMI, the largest three pharmaceutical wholesalersCardinal Health, Cencora, and McKessonare using vertical integration to build significant market positions in businesses beyond drug distribution. This video was excerpted from my recent Drug Channels Outlook 2025 webinar.

Drug Channels
DECEMBER 9, 2024
This week, Im rerunning some popular posts while I prepare for Fridays Drug Channels Outlook 2025 live video webinar. During Friday's webinar, Ill share some updated thoughts on biosimilars and PBMs private label products. The Humira biosimilar market just took another step forwardbut remains far from its ideal state.

Drug Channels
FEBRUARY 4, 2025
ICYMI, the largest three pharmaceutical wholesalersCardinal Health, Cencora, and McKessonare using vertical integration to build significant market positions in businesses beyond drug distribution. Outline the market access implications for provider-administered biosimilars in the buy-and-bill market. All rights reserved.

FDA Law Blog: Biosimilars
APRIL 8, 2025
The DCAP was an initiative launched in 2017 to remove barriers to generic drug development, approval, and market entry (see our previous post here ). This facilitated generic manufacturers access to samples of brand-name drugs not readily available through normal market channels for generic drug development.

FDA Law Blog: Biosimilars
MARCH 9, 2025
The American Conference Institutes 12th Annual Legal, Regulatory, and Compliance Forum on Cosmetics & Personal Care is scheduled to take place from March 27-28, 2025, at the New York City Bar Association, New York, NY. FDA Law Blog is a conference media partner. As such, we can offer our readers a special 10% discount.

FDA Law Blog: Biosimilars
NOVEMBER 11, 2024
Comments on the proposed order are due May 25, 2025. Nor does it allow for full consideration of the implications of removing PE from the monograph and leaving pseudoephedrine as the only oral decongestant active ingredient that can be used in an OTC drug marketed under the monograph.

FDA Law Blog: Biosimilars
JANUARY 15, 2025
Koblitz On January 7, 2025, FDA announced that back on November 12, 2024, the Center for Veterinary Medicine (CVM) issued Warning Letters to six online retailers marketing unapproved new animal drug products that purported to treat and control seizures and epilepsy in dogs and cats. By Charles D. Snow & Sara W. see also id.

FDA Law Blog: Biosimilars
NOVEMBER 7, 2024
If it does, the letter will also identify the device class, whether a premarket notification or premarket approval is required to market the device, and other pertinent information. For fiscal year 2025, which began on October 1 and runs through September 30, 2025, the standard fee is $7,301.

FDA Law Blog: Biosimilars
APRIL 6, 2025
2, 2025) that affirms FDAs denial of authorization to market flavored vape products. The Court remanded the case to the Fifth Circuit to determine whether FDA had engaged in harmless error when it abandoned a prior requirement that manufacturers submit its marketing plans for their products. 23-1038 (Apr.

FDA Law Blog: Biosimilars
MARCH 30, 2025
Livornese & Ricardo Carvajal On March 18, 2025, the U.S. Infant Formula Market that FDA announced in January. By Charles D. Snow & Deborah L. Curiously, the announcements make no reference to the Long-Term National Strategy to Increase the Resiliency of the U.S.

FDA Law Blog: Biosimilars
JANUARY 16, 2025
Hyman, Phelps & McNamara PC, (HPM), which will mark its 45thAnniversary on March 17, 2025, is pleased to announce that it is increasing its directors, counsel, and associates as it starts the year. Richardson has been promoted to Director.

FDA Law Blog: Biosimilars
JANUARY 5, 2025
Livornese As promised in the Fall Unified Regulatory Agenda, FDA issued the final rule to establish the pathway to obtain marketing approval of a nonprescription drug product with an additional condition for nonprescription use (ACNU) on December 26, 2024, before the end of the calendar year. By Deborah L. 105288 (Dec.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content