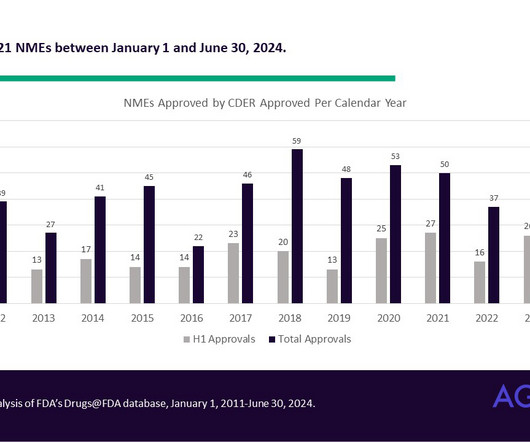
Q&A with Worldwide’s Rare Disease Experts: The Latest Innovations and Hopes for 2025
Conversations in Drug Development Trends
NOVEMBER 5, 2024
In preparation for World Orphan Drug Congress Europe, we interviewed Nathan Chadwick, Senior Director, Therapeutic Strategy Lead, Rare Disease, and Derek Ansel, MS, LCGC, Vice President, Therapeutic Strategy Lead, Rare Disease, to hear their insights into the current progress in rare disease research and their hopes for 2025.






























Let's personalize your content