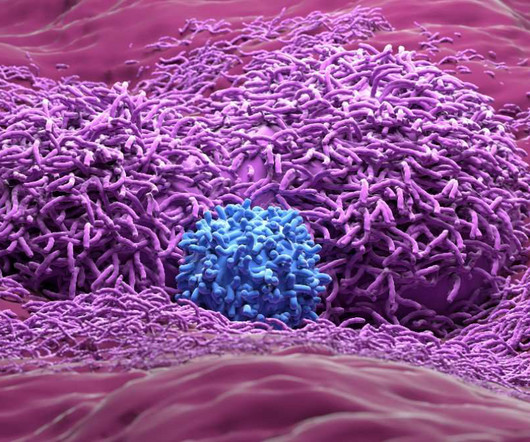
FDA clears genetically engineered TIL therapy for solid tumour trials
Drug Discovery World
SEPTEMBER 3, 2024
The therapy is now approved by both the FDA and China Center for Drug Evaluation (CDE) to enter clinical trials in both countries for advanced solid tumour patients. Diana Spencer, Senior Digital Content Editor, DDW The post FDA clears genetically engineered TIL therapy for solid tumour trials appeared first on Drug Discovery World (DDW).



































Let's personalize your content