Antibody Drug Conjugates: windows of opportunity
Drug Target Review
JULY 4, 2023
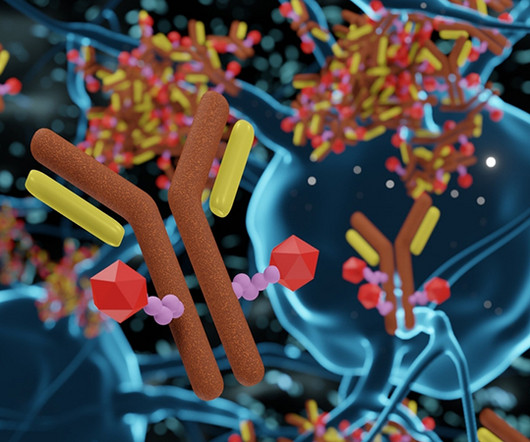
These therapies have broadened treatment options for patients to expand beyond the more traditional small molecule drug alternatives. ADCs have the potential to redress the poor balance between safety and efficacy seen with traditional cancer treatment options. 3D rendering of Antibody Drug Conjugate Molecules.













Let's personalize your content