Gilead expands Arcellx cancer cell therapy deal
BioPharma Drive: Drug Pricing
NOVEMBER 15, 2023
The deal gives Gilead an estimated 13% ownership in Arcellx and extends the company’s cash runway into 2027.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
 2027 Related Topics
2027 Related Topics 
BioPharma Drive: Drug Pricing
NOVEMBER 15, 2023
The deal gives Gilead an estimated 13% ownership in Arcellx and extends the company’s cash runway into 2027.

BioPharma Drive: Drug Pricing
FEBRUARY 6, 2025
The pharmaceutical company plans to cut $2 billion in annual expenses by the end of 2027 through “operational efficiencies across multiple areas of the business,” its CFO told analysts on a conference call.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The Pharma Data
FEBRUARY 8, 2021
RE47,739 (‘739) by more than four years until March 5, 2027. Patent Term Extension (PTE) certificate for IBRANCE® (palbociclib). The certificate extends the term of U.S. The PTE certificate was granted under the patent restoration provisions of the Drug Price Competition and Patent Term Restoration Act of 1984.
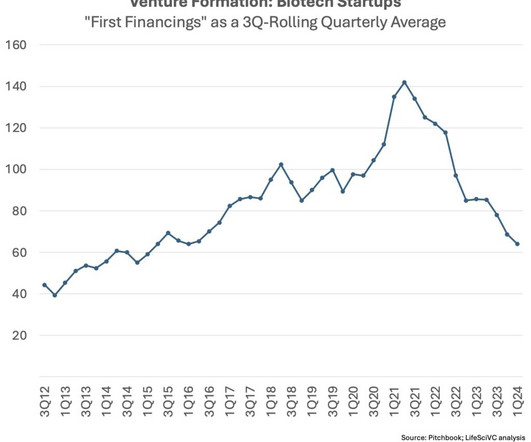
LifeSciVC
APRIL 26, 2024
Fourth, the venture exit environment circa 2027-2030 for the new crop of early stage startups being created today will likely have structural supply/demand elements more favorable than the backdrop in recent years. Fewer startups over time should ameliorate this crowding dynamic to some extent.

Tecan
DECEMBER 7, 2022
By Joe Guterl More than $72 billion – that is what some researchers estimate will be the global point-of-care (POC) biochemical diagnostic testing market size in 2027, up from $36 billion in 2021.

FDA Law Blog: Biosimilars
MAY 13, 2024
Further, FDA has stated that enabling advanced data analytics, including RWD, is one of the objectives incorporated in the FDA’s Information Technology Strategy for FY 2024-2027. We, and many stakeholders, agree that RWD and RWE can play an invaluable role.

thought leadership
MAY 1, 2023
billion USD by 2027, with the goal of improving the generation, collation, storage, and utilization of data 1. Investments in digital transformations are expected to grow from $594.5 billion USD in 2022 to $1.548.9 This drive to better manage and protect data is not a luxury.

FDA Law Blog: Biosimilars
JUNE 8, 2023
FDA notes that all in-person FTF formal meetings at CDER and CBER will have a hybrid component (virtual attendees in addition to in-person attendees).

Agency IQ
JULY 22, 2024
The independent auditing schedule showed publication of full functionality in mid-2027. The first five modules would be audited between Q2 2024 and Q1 2025 and the CI/PS module between Q4 2026 and Q1 2027, with publication of complete functionality occurring by the end of Q2 2027. There’s a notable absence from this roadmap.

FDA Law Blog: Biosimilars
OCTOBER 6, 2023
The Proposed Rule states that Stage 4 and Stage 5 would not begin before October 1, 2027, and April 1, 2028, respectively, in order to enable laboratories to participate in negotiations preceding user fee reauthorization in 2027 (taking effect in FY2028, which begins on October 1, 2027).

FDA Law Blog: Biosimilars
SEPTEMBER 16, 2024
This FR Notice and draft strategy document are part of FDA’s commitment under the Prescription Drug User Fee Act (PDUFA) Reauthorization Performance Goals and Procedures for Fiscal Years 2023-2027 (PDUFA VII), wherein FDA committed to advance the use and implementation of innovative manufacturing.
The Pharma Data
SEPTEMBER 27, 2021
5.500% Notes due 2027 532457 AZ1 7 0.750% due August 31, 2026 0.820% 40 bps $1,226.23 3.100% Notes due 2027 532457 BP2 13 0.750% due August 31, 2026 0.820% 35 bps $1,100.66 5.500% Notes due 2027 532457 AZ1 7 0.750% due August 31, 2026 0.820% 40 bps $1,226.23
The Pharma Data
SEPTEMBER 7, 2021
5.500% Notes due 2027. 3.100% Notes due 2027. 284,112,000. 1.750% due August 15, 2041. 5.550% Notes due 2037. 532457 BA5. 476,152,000. 1.750% due August 15, 2041. 532457 AZ1. 377,505,000. 0.750% due August 31, 2026. 4.650% Notes due 2044. 532457 BG2. 43,016,000. 1.750% due August 15, 2041. 3.950% Notes due 2047. 532457 BR8. 532457 BV9.
Drug Target Review
JUNE 5, 2023
How does Zymeworks plan to advance at least five novel medicines into clinical studies by 2027 under its ZYME 5×5 R&D objectives? We continue our in-house development strategy of accelerating our time from preclinical development candidate selection through IND filing.

Perficient: Drug Development
AUGUST 12, 2023
The AI market is projected to reach $407B by 2027, which is substantial growth from its $86.9B This is what everyone is talking about in the market, and I couldn’t be more excited to learn not just about Salesforce AI capabilities, but how we can understand and trust AI more effectively. in revenue in 2022.

The Pharma Data
MAY 2, 2022
Amgen made continued progress last year toward its goal of achieving carbon neutrality in our operations by 2027 3. A Healthy Amgen: We hold ourselves to high?standards standards in our?operations operations – working to?ensure ensure our actions and culture reflect Amgen?values.

Alta Sciences
JANUARY 31, 2025
Ria is currently an industry member of the STCs Scientific Program Committee and will serve in this role until 2026, after which she will transition to Committee Chair until 2027. Image Thumbnail_Blog_RiaFalvo-V2.jpg

Perficient: Drug Development
DECEMBER 27, 2023
The effective date of the new rule is April 1, 2024, with key provisions taking effect on January 1, 2026, and January 1, 2027. Banks Additionally, the recent rule by the OCC, the Federal Reserve, and the FDIC strengthens and modernizes Community Reinvestment Act (CRA) regulations.

FDA Law Blog: Biosimilars
OCTOBER 12, 2023
Priority is given to Suitability Petitions that could mitigate or resolve a drug shortage; address a public health emergency declared by HHS; mitigate waste by way of new strengths for parenteral products; or subject to special review under the President’s Emergency Plan for Aids Relief.

FDA Law Blog: Biosimilars
NOVEMBER 22, 2023
Moreover, California recently enacted the California Food Safety Act , prohibiting the manufacturing, selling, delivering, distributing, or holding food that contains BVO, with a $5,000 civil penalty for first violations, as of January 1, 2027. In addition, New York introduced a similar bill prohibiting certain food additives, including BVO.

The Premier Consulting Blog
OCTOBER 1, 2023
PDUFA VII will be in effect from fiscal year 2023 through fiscal year 2027. It is the sixth reauthorization of PDUFA that provides the FDA with necessary resources and includes provisions to increase the efficiency of the drug development process. Check out our previous blog post to learn more about changes that came in PDUFA VII.

FDA Law Blog: Biosimilars
JUNE 14, 2023
In FY 2026 – FY 2027, continue to support products enrolled in previous fiscal years and expand to enroll up to 100 additional products each fiscal year within existing OHTs or expand to additional OHTs, depending on lessons learned from FY 2023 – FY 2025 experience (i.e., up to 125 total products enrolled through FY 2025).

The Pharma Data
FEBRUARY 22, 2022
In January 2021, Amgen announced plans to achieve carbon neutrality in its operations by 2027 , while further reducing water use by 40% and waste disposed by 75%. 1 These reductions were achieved even as the Company increased production capacity, expanded its presence to approximately 100 countries, and grew revenues significantly.

The Pharma Data
NOVEMBER 29, 2021
” Amgen’s four ESG pillars – Healthy People, Healthy Society, Healthy Environment and a Healthy Amgen – include commitments to: Achieve carbon neutrality by 2027, along with a 40% reduction in water used and a 75% reduction in waste disposed.

Agency IQ
JANUARY 26, 2024
Class A devices needed to be IVDR-ready on the original date (May 26, 2022), but class D had until May 2025, class C until May 2026, and class B and sterile class A until May 2027. In-house tests (in the U.S. called laboratory-developed tests manufactured and used in a single lab) had until May 26, 2024.

FDA Law Blog: Biosimilars
AUGUST 23, 2023
A DHT is considered “a system that uses computing platforms, connectivity, software, and sensors for healthcare and related uses.”

The Pharma Data
SEPTEMBER 27, 2021
Acceptance Priority Level Principal Amount Outstanding Principal Amount Tendered Approximate Percentage of Outstanding Amount Tendered Anticipated Principal Amount to be Accepted for Purchase 4.150% Notes due 2059 532457 BU1 1 (1) $1,000,000,000 $408,714,000 40.87% $408,714,000 3.950% Notes due 2049 532457 BT4 2 (2) $1,500,000,000 $541,847,000 36.12% (..)

The Pharma Data
DECEMBER 30, 2020
billion by 2027. Propanc Biopharma – Australia-based Propanc Biopharma announced the Proenzymes Optimization Project 1 (POP1) joint research and drug discovery program advanced towards producing commercial scale quantities of the two proenzymes trypsinogen and chymotrypsinogen.

The Pharma Data
MARCH 7, 2022
The new facility will also be built to exacting environmental standards, consistent with the company’s goal of reducing water usage and waste and achieving carbon neutrality by 2027. Construction management and design services for this new state-of-the-art facility are being led by Integrated Project Services, LLC.

The Pharma Data
JUNE 26, 2021
To that order and to minimize the potential direct and indirect impacts of its business on the environment throughout the whole lifecycle of its products, the company intends to: protect ecosystems by introducing biodiversity protection plans at all its sites located near sensitive areas by 2025; implement water stewardship and water efficiency plans (..)

The Pharma Data
DECEMBER 14, 2020
Market researchers expect the global market for gene and cell therapy to grow at a CAGR of over 25% through 2027. In the medium term, however, c-LEcta’s management expects demand to develop dynamically, especially from gene and cell therapy. The DENARASE ELISA kit was also launched in 2020 to complement the company’s current products.

The Pharma Data
APRIL 7, 2021
To reduce its greenhouse gas emissions by 55% by 2030 and contribute to better resource conservation, Sanofi plans to remove all pre-formed plastic packaging (blister packs) for its vaccines by 2027. The company is also committed to ecodesigning all its new products by 2025.

The Pharma Data
FEBRUARY 18, 2022
” Amgen’s four ESG pillars – Healthy People, Healthy Society, Healthy Planet, and a Healthy Amgen – include commitments to: Achieve carbon neutrality by 2027, along with a 40% reduction in water used and a 75% reduction in waste disposed.

The Pharma Data
JANUARY 26, 2021
billion by 2027. . With very limited approved first-line pharmaceutical therapies for HCC available today, challenges include drug resistance, adverse side effects, and high costs. An estimated 700,000 people are diagnosed with HCC each year, with the global market for liver cancer drugs expected to grow to $3.9

FDA Law Blog: Biosimilars
JUNE 7, 2023
Recall that CMS will require manufacturers of selected drugs to negotiate a maximum fair price (“MFP”) with the agency according to the following timeline (the dates apply from applicable year 2027 onwards).

The Pharma Data
JANUARY 25, 2021
This activity could increase (or reduce the size of any decrease in) the market price of the Company’s common stock, the notes or the Company’s 2.50% Convertible Senior Notes due 2027 at that time.

Advarra
JUNE 13, 2023
between 2020-2027. Per a report from the American Society of Cell and Gene Therapy (ASGCT) , gene therapy research is an increasingly important part of an institution’s research portfolio, with over 3,500 therapies in preclinical or clinical. The accelerating gene therapy market is expected to grow globally by 16.6%

The Premier Consulting Blog
APRIL 27, 2023
How can you prepare?

Vial
FEBRUARY 29, 2024
The Clinical Trials Outsourcing Market – Global Outlook & Forecast 2022-2027 report lists the following key trends in the future of the CRO global market. A rise of clinical trial outsourcing due to cost management, globalization, and digitalization.

FDA Law Blog: Biosimilars
MARCH 5, 2024
To the extent that AstraZeneca alleged that CMS would cause it this type of harm in 2027, the court found that allegation to be too speculative. Because the alleged harm arises from the Act, not from the Guidance, the court found that AstraZeneca did not meet the causation or redressability elements of standing under this argument.

FDA Law Blog: Biosimilars
JULY 24, 2023
With that decision, Jazz lost its ODE, allowing Avadel’s sodium oxybate product to compete with Jazz’s long before the expiration of the ODE covering Jazz’s most recent product, Xywav, in 2027.

The Pharma Data
JUNE 7, 2022
Those taking up this offer will be required to hold the Shares or the corresponding FCPE units for a period of approximately five years, i.e. until 31 May 2027, except upon the occurrence of an early release event provided for under Article R. The voting rights attached to the subscribed Shares will be exercised directly by the employees.

Vial
DECEMBER 7, 2023
billion by 2027 , at a compound annual growth rate (CAGR) of 45.7% As a newer alternative to the time-consuming nature of traditional candidate screening and drug discovery, AI-designed drugs have started to make their mark, promising quick and efficient results. billion in 2022 and is expected to grow to US$4.0 between these years.

Agency IQ
MAY 17, 2024
GDUFA III), which reauthorized the program through 2027. The generic drug user fee program is currently on its third iteration (i.e., Under GDUFA III, the Agency agreed to improve and expand upon the various meeting types which generic drug developers can take advantage of to discuss issues relevant to their development programs with FDA.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content