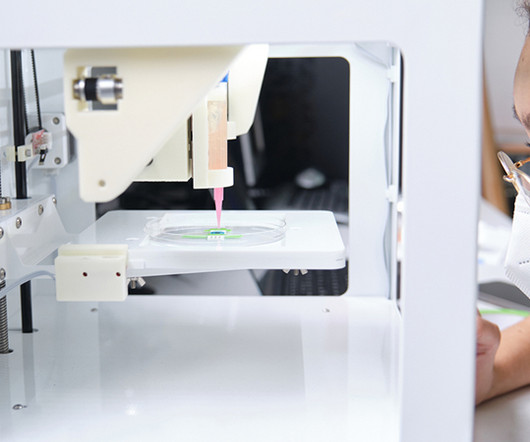
Exploring alternatives to animal testing in drug discovery
Drug Target Review
JULY 6, 2023
Animal testing plays a significant role in pre-clinical research and therefore requires the use of millions of animals. million scientific procedures involving live animals were carried out in 2020. million scientific procedures involving live animals were carried out in 2020. In Britain, 2.88 In Britain, 2.88

























Let's personalize your content