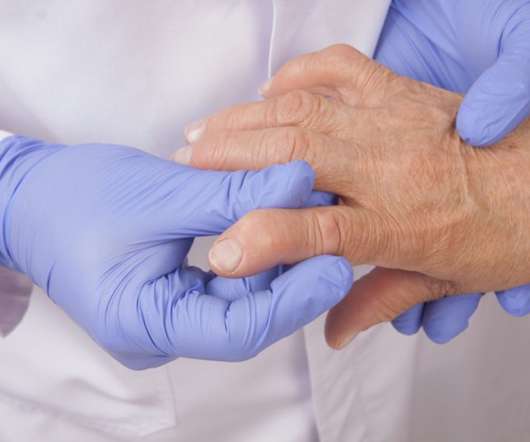
COMMERCIALIZATION AND LICENSE AGREEMENT FOR PROPOSED BIOSIMILAR CURRENTLY IN PHASE 3 WITH THE POTENTIAL TO TREAT MODERATE TO SEVERE RHEUMATOID ARTHRITIS
The Pharma Data
APRIL 9, 2021
Biogen enters into a commercialization and license agreement to develop, manufacture and commercialize BAT1806, a proposed biosimilar referencing ACTEMRA ® (tocilizumab). Biosimilars have the potential to enable greater access to marketed biologic therapies while generating cost savings and healthcare sustainability.















































Let's personalize your content