Four Crucial Questions about the Humira Biosimilar Price War (rerun)
Drug Channels
OCTOBER 3, 2023
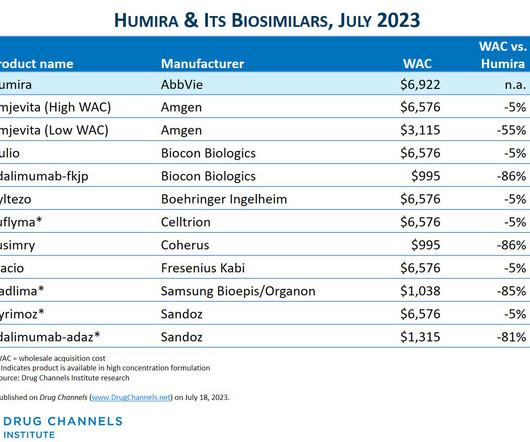
This week, I’m rerunning some popular posts while I put the finishing touches on our new 2023-24 Economic Report on Pharmaceutical Wholesalers and Specialty Distributors. Boehringer-Ingelheim launched an unbranded, low WAC version of its interchangeable biosimilar. The Humira biosimilar market has arrived!





























Let's personalize your content