Drug Discovery Industry Roundup with Barry Bunin — March 1, 2024
Collaborative Drug
MARCH 1, 2024
FDA Approved 55 New Molecular Entities in 2023 Can Ancient Skeletons Give Clues to Modern Medical Mysteries? and More
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Collaborative Drug
MARCH 1, 2024
FDA Approved 55 New Molecular Entities in 2023 Can Ancient Skeletons Give Clues to Modern Medical Mysteries? and More
Metabolite Tales Blog
MAY 3, 2023
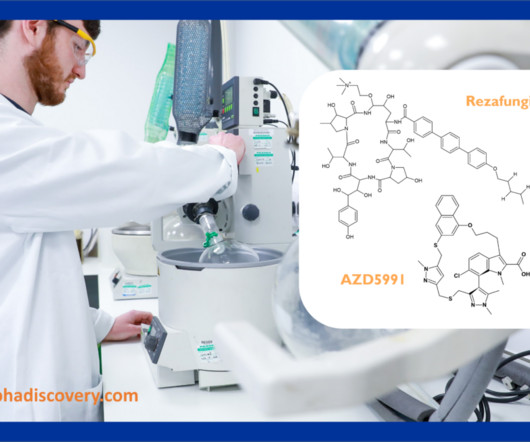
Hypha’s Q2 2023 Newsletter – acyl glucuronides, hydroxylated metabolites and our latest blogs In our Q2 2023 newsletter we look at the synthesis of a prominent acyl glucuronide of the Mcl-1 inhibitor AZD5991, hydroxylated metabolites of the recently FDA approved rezafungin, as well as links to our latest blogs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Collaborative Drug
OCTOBER 7, 2024
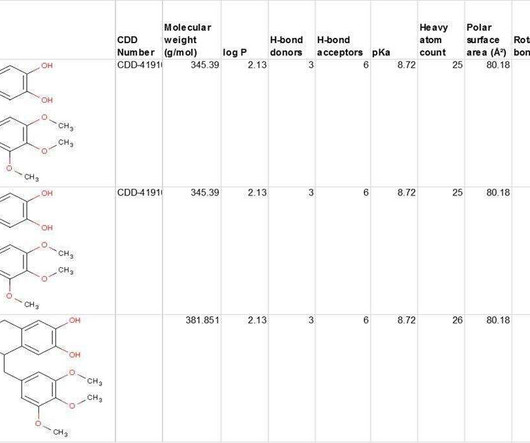
CDD Vault provides conventional SAR tables of course, but it also gives you access to data from multiple public sources for comparison with hundreds of published sources, including popular MLSMR, GlaxoSmithKline TCAMs, and FDA-Approved Re-purposed Drugs data sets.

Metabolite Tales Blog
APRIL 10, 2024
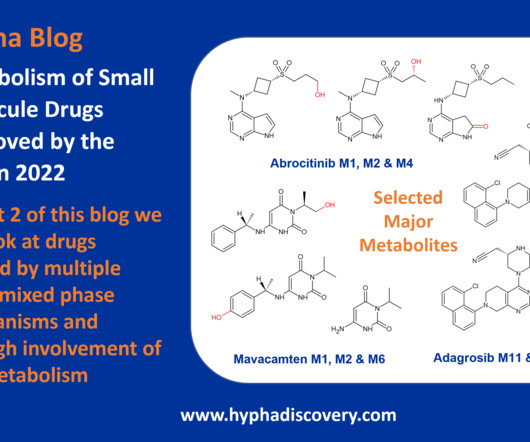
It is approved for treatment of severe alopecia areata in which inflammatory processes play a major role, with associated increased reactive oxygen species formation and reduced levels of GSH. The next blog will complete our commentary on metabolism of small molecule drugs approved in 2023. Clin Pharmacokinet.
Metabolite Tales Blog
APRIL 4, 2023
Metabolism of 2022 FDA approved small molecule drugs part 2 Mixing it Up By Julia Shanu-Wilson In Part 1 of this topic we looked at metabolism of the small molecule drugs approved by the FDA in 2022 that were mediated by CYP3A4. Dermavant’s tapinarof is one such friend. 8 This is not the only point of interest.
Metabolite Tales Blog
JANUARY 26, 2023
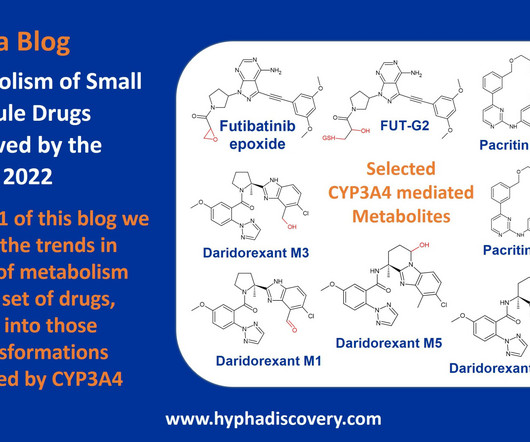
Metabolism of 2022 FDA approved small molecule drugs – Part 1 Does CYP3A4 still rule? By Julia Shanu-Wilson It won’t come as much surprise to learn that of the 17 small molecules* approved by the FDA in 2022, CYP3A4 was the major player in drug metabolism. References Iversen et al., Front Pharmacol.,

FDA Law Blog: Biosimilars
MARCH 29, 2023
Richardson — Early on March 29, 2023, FDA announced the landmark approval of Narcan (naloxone hydrochloride) Nasal Spray for use as a nonprescription opioid overdose reversal agent. Today’s OTC naloxone approval is limited to NNS specifically, but we are aware of other OTC naloxone applications currently being reviewed by FDA.

FDA Law Blog: Drug Discovery
NOVEMBER 10, 2024
The rest of this blog will focus on the Clinical Trials Grants Program. The FDA’s Orphan Products Clinical Trials Grants Program is open to academic institutions, industry sponsors, non-profit organizations, and public or private entities, both within and outside the U.S. Relative to other areas of medicine (e.g.,
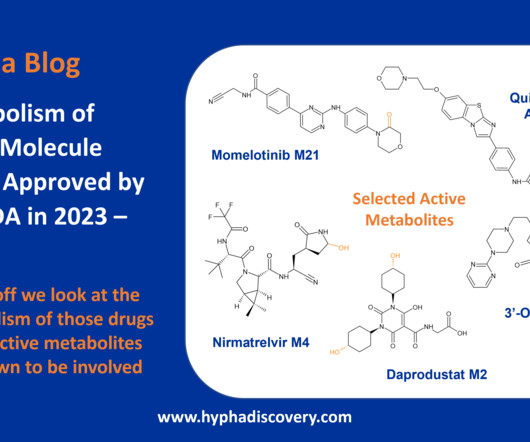
Metabolite Tales Blog
JANUARY 23, 2024
Metabolism of 2023 FDA Approved Small Molecules – PART 1 By Julia Shanu-Wilson 2023 was a fruitful year for drug approvals by the FDA, with a crop of 34 small molecules out of a total of 55 new drugs [1]. link] [19] FDA prescribing information for zilucoplan.

FDA Law Blog: Biosimilars
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts. Mensing, there was much discussion about adding authority to the FDC Act to allow generics to make safety changes to product labeling without FDA approval.

FDA Law Blog: Drug Discovery
MARCH 26, 2024
We authors eagerly await the publication of FDA’s Summary Basis for Approval for the Duvyzat NDA so that we can better understand the basis for the Agency’s finding of substantial evidence of effectiveness, particularly in light of recent FDA guidance on the topic of single study approvals with confirmatory evidence, available here.

National Institute on Drug Abuse: Nora's Blog
FEBRUARY 1, 2024
50 years after founding, NIDA urges following science to move beyond stigma area Thu, 02/01/2024 - 11:20 Nora's Blog February 1, 2024 Image NIDA Image In 2024, NIDA celebrates its 50th anniversary. People in rural communities may have particular difficulty accessing care. Most of the fatal overdoses each year involve opioids.

FDA Law Blog: Biosimilars
JUNE 27, 2023
Amongst his accomplishments, Law360 considered the role James has played in leveraging little-used pathways to FDA approval for often first-ever drugs to treat rare diseases (e.g., James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

Pharmaceutical Development Group
JANUARY 7, 2022
Effective and consistent use and application of Data Standards can reduce costs of Pharmaceutical Drug and Biologic Products and Process Development, Drug Development Services, 505(b) NDA, IND Consulting , NDA Consulting, BLA Consulting , and effective FDA Pre-Submission collectively resulting in FDA Approval. Spanogle, Ph.D.

Pharmaceutical Development Group
DECEMBER 19, 2021
Effective use of Real World Data (RWD) and Real World Evidence (RWE) can reduce costs of Pharmaceutical Drug and Biologic Products and Process Development, Drug Development Services , expedite a FDA Pre-Submission Review, and lead to FDA Approval. Author Information William E. Spanogle, Ph.D.
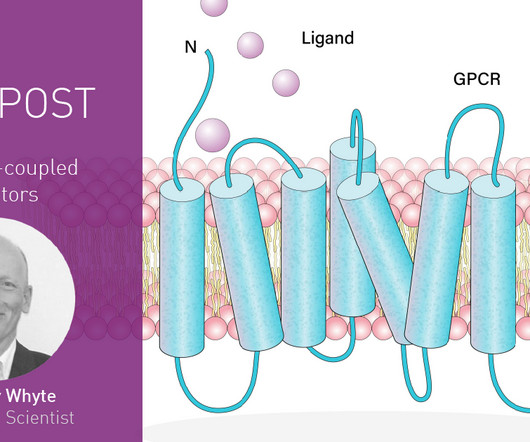
BMG Labtech
JANUARY 18, 2023
And if you need to take an FDA-approved drug, there’s around a one in three chance that it’s a drug that targets a GPCR. In the first part of this blog article, we look at what GPCRs are, some of their responsibilities in the cell, and the overall approaches used to study these signaling molecules.

FDA Law Blog: Biosimilars
FEBRUARY 27, 2024
Karst — It’s been a while since we last blogged on Patent Term Extension (“PTE”) issues of interest. 156, a patent may be extended only once (even if it would be eligible for extension on more than one occasion because it applies to several FDA-approved products), and only one patent may be extended for each regulatory review period.

FDA Law Blog: Biosimilars
MAY 11, 2023
Koblitz — Well, we’re a little late to blogging about this, but the significance of the ongoing Teva v. GSK litigation to Hatch-Waxman aficionados makes this case still ripe for blogging. government in the now-infamous (at least in FDA circles) Teva v.

EG Life Sciences
FEBRUARY 15, 2023
The US Food and Drug Administration (FDA) approved around 26 novel drugs in 2022. Approval processes were slow in comparison to 2020 and 2021, where 53 and 50 approvals were achieved respectively. Going into the third year of the pandemic, we know that the virus still impacts industry operations.

New Drug Approvals
DECEMBER 19, 2022
FDA 12/1/2022, To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, Rezlidhia Olutasidenib , sold under the brand name Rezlidhia , is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia. [1] 1] It is taken by mouth. [1] Hz, 1 H), 4.62−4.75

FDA Law Blog: Biosimilars
JANUARY 4, 2024
The American Conference Institute (“ACI”) will be hosting the go-to forum for critical updates on OTC regulation and enforcement, monograph reform, ACNU and advertising essentials… and FDA Law Blog readers can get a discount. FDA Law Blog is a conference media partner for this event.

FDA Law Blog: Biosimilars
JUNE 13, 2024
FTC, deep in its foray into the Orange Book, filed an Amicus Brief in the case arguing that the patents do not claim any FDA-approved drug.

FDA Law Blog: Biosimilars
AUGUST 7, 2024
ENTRESTO was approved by FDA in July 2015 “to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.”

DS in Pharmatics
OCTOBER 14, 2022
An Investigational Device Exemption (IDE) is an application submitted to obtain the FDA's approval for use of a novel medical device in a clinical study. This allows for the collection of safety and effectiveness data in order to support full market approval. IDE applications support several types of studies: to […]
PerkinElmer
AUGUST 13, 2020
The virus, which can cause a severe form of pneumonia and lead to acute respiratory distress, currently has no FDA-approved targeted therapeutic or vaccine. Sources [link] [link] The post Use of Ultra-High-Throughput Screening in Discovery of COVID-19 Virus Structure first appeared on PerkinElmer Blog.

National Institute on Drug Abuse: Nora's Blog
APRIL 10, 2023
mfleming Mon, 04/10/2023 - 14:43 Nora's Blog April 13, 2023 Image Getty Images/ AleksandarGeorgiev This article originally appeared in the Milken Institute’s Power of Ideas series. Is It Too Soon To Start Talking about a Cure for Addiction? Our country remains in the grips of an opioid crisis claiming more than 100,000 lives every year.
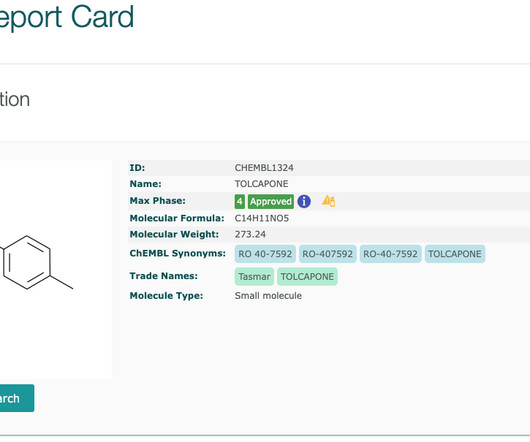
The ChEMBL-og
FEBRUARY 22, 2021
Boxed warnings (also know as black box warnings) are provided on medicinal product labels for FDA approved drugs if the medicinal product can cause severe or life-threatening side effects. See our blog on Withdrawn Drugs. They are free text descriptions, enclosed within a black box, hence the name!

Common Sense for Drug Policy Blog
MAY 1, 2024
However, prescription drugs must be approved by the Food and Drug Administration (FDA). Although FDA has approved some drugs derived from or related to cannabis, marijuana itself is not an FDA-approved drug.

FDA Law Blog: Biosimilars
APRIL 21, 2024
Karst — If you’ve been following this blog since the early days, then you know we fervently followed the more-than-decade-long soap opera that was The Medicines Company’s efforts to obtain a Patent Term Extension (“PTE”) from the U.S. Usually at this point in a post we would identify the date of approval of the relevant NDA.

The Premier Consulting Blog
DECEMBER 28, 2023
Clinical studies are often conducted to support a Premarket Approval (PMA) application, though some 510(k) submissions may require clinical data. In this blog post, we discuss key considerations for assessing the need for an IDE and complying with the reporting requirements under the IDE program.

FDA Law Blog: Biosimilars
JANUARY 18, 2024
FDA has not approved an NDA for a drug containing botanical marijuana but notes that two drug products containing delta-9-tetrahydrocannabinol (“delta-9-THC”) (as dronabinol), the primary compound in marijuana have received FDA approval: Marinol and Syndros. chemotherapy-induced), and pain.

New Drug Approvals
SEPTEMBER 20, 2023
1] Motixafortide was approved for medical use in the United States in September 2023. [2] 4 Similar in mechanism to the previously approved plerixafor , motixafortide is an inhibitor of C-X-C Motif Chemokine Receptor 4 (CXCR4), a protein that helps to anchor stem cells to bone marrow matrix. 1] It is given by subcutaneous injection. [1]

Alta Sciences
FEBRUARY 8, 2024
Image Blog-Thumbnail_Driving-sim-webinar.jpg Synopsis Learn more about how Altasciences can test cognition, and the impact of driving under the influence of medication, using our state-of-the-art driving simulators. View the Driving Simulation Fact Sheet for more information.

The Premier Consulting Blog
APRIL 15, 2024
In this blog, we will go into more detail about the unwritten nonclinical requirements for the PIND meeting.

Molecule Blog
AUGUST 13, 2021
If successful, this work could result in the repurposing of several FDA-approved therapeutics for the purpose of extending the human lifespan, at a lower cost and over faster timelines than conceivably possible with de novo drug discovery. The Scheibye-Knudsen lab has analyzed 1.5 billion prescriptions from 4.8

FDA Law Blog: Biosimilars
JANUARY 18, 2024
Palmer — A new lawsuit against FDA is the latest happening in the veterinary drugs space and, by extension, FDA’s Center for Veterinary Medicine (CVM). We blogged about CVM last week and explained the increasing attention to animal health products due to the expansion of the animal and pet product market. Koblitz & Karla L.

KIF1A
APRIL 25, 2024
We are quickly approaching Sloane’s ASO dosing and I thought it might be helpful to “blog” about our experience so you all can follow along to see what the process is like. However, we needed to wait for Susannah to finish her trial before we could submit to the FDA for Sloane’s ASO use. Hello KIF1A Community!

DS in Pharmatics
NOVEMBER 16, 2023
Proactive vendor evaluations, comprehensive domain expertise, and FDA-approved audits – DSI is your trusted partner for seamless 2024 GCP GMP audits.

DS in Pharmatics
NOVEMBER 16, 2023
Proactive vendor evaluations, comprehensive domain expertise, and FDA-approved audits – DSI is your trusted partner for seamless 2024 GCP GMP audits.

PerkinElmer
APRIL 21, 2020
Using the isolated ZIKV MEX_I_7 strain, researchers got a head start on potential leads by interrogating a library of existing, FDA-approved drugs that can be used off-label for treatment of Zika. High-content imaging was used in a recent study aiming to identify novel ZIKV inhibitors from a pool of 774 compounds.

PerkinElmer
JANUARY 7, 2022
link] New safety concerns identified for 1 in 3 FDA-approved drugs [Internet]. Available from: [link] The post Improving Drug Safety Through Cardiotoxicity Assessment first appeared on PerkinElmer Blog. Improving the odds of drug development success through human genomics: modelling study. Sci Rep 9, 18911 (2019).

New Drug Approvals
DECEMBER 24, 2023
3] In November 2023, capivasertib was approved in the United States for people with hormone receptor-positive, human epidermal growth factor receptor 2 -negative breast cancer when used in combination with fulvestrant. [3] Jump up to: a b c d e f “FDA approves capivasertib with fulvestrant for breast cancer” U.S.

FDA Law Blog: Biosimilars
MARCH 3, 2024
Effect on FDA approvals— In cases where a biological product or drug needs to change aspects of its manufacturing processes to avoid using a covered equipment or service, will it need to file supplements with FDA for the CMC update?
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content