ChEMBL 34 is out!
The ChEMBL-og
APRIL 16, 2024
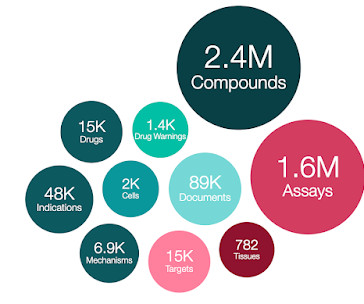
We are delighted to announce the release of ChEMBL 34, which includes a full update to drug and clinical candidate drug data. 71 out of the 882 newly added EMA drugs are only authorised by EMA, rather than from other regulatory bodies e.g. FDA. This data is currently available for European Medicines Agency drugs only.







Let's personalize your content