Evolution of Clinical Operations Drives FSP Staffing Models to the Forefront
PPD
OCTOBER 2, 2024
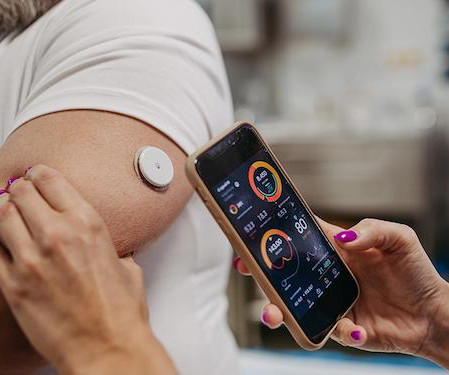
Efficient and effective clinical operations are the backbone of successful clinical trials, and today’s biopharmaceutical, biotech and medical device organizations have a range of options to meet their needs in this critical area.










Let's personalize your content