Regulator and Funder? FDA’s Orphan Products Grants Program awards significant funding to help move promising treatments through clinical development
FDA Law Blog: Drug Discovery
NOVEMBER 10, 2024
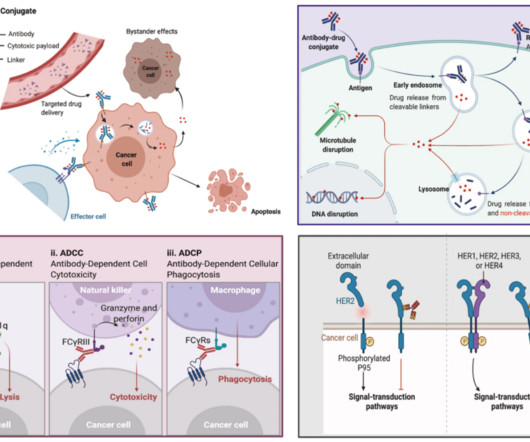
Food and Drug Administration (FDA) plays a pivotal role in fostering the development of treatments for rare diseases through its Orphan Products Grants Program. Each year, FDA selects a limited number of clinical trials to fund to help sponsors pursue development of medical products for rare diseases and advance their field.












































Let's personalize your content