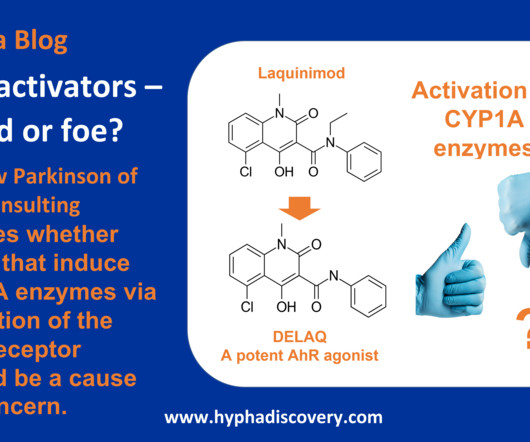
J&J presents successful data from plaque psoriasis trial
Drug Discovery World
MARCH 13, 2024
In the five JNJ-2113 treatment groups, as measured by the Psoriasis Area and Severity Index (PASI), response rates were maintained from Week 16 to Week 52, with the highest PASI 75 response observed in the 100mg twice daily group (78.6 Across JNJ-2113 treatment groups, 58.6% at 16 weeks and 76.2% at 16 weeks and 76.2% at 52 weeks).













































Let's personalize your content