Metabolism of 2022 FDA approved small molecule drugs PART 2
Metabolite Tales Blog
APRIL 4, 2023
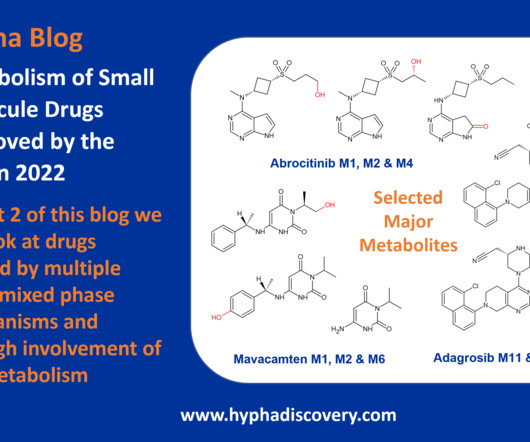
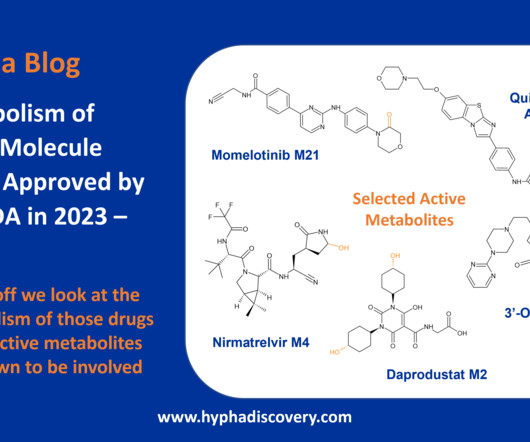
Metabolism of 2022 FDA approved small molecule drugs part 2 Mixing it Up By Julia Shanu-Wilson In Part 1 of this topic we looked at metabolism of the small molecule drugs approved by the FDA in 2022 that were mediated by CYP3A4. We hope it was a useful two-parter! We’re looking forward to the next crop! Acta Pharm Sin B.



















Let's personalize your content