Metabolism of 2023 FDA Approved Small Molecules – PART 1
Metabolite Tales Blog
JANUARY 23, 2024
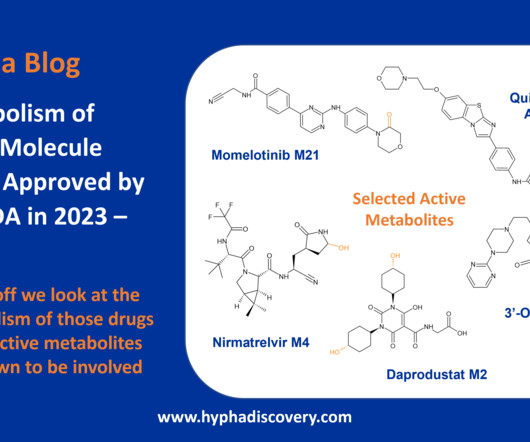
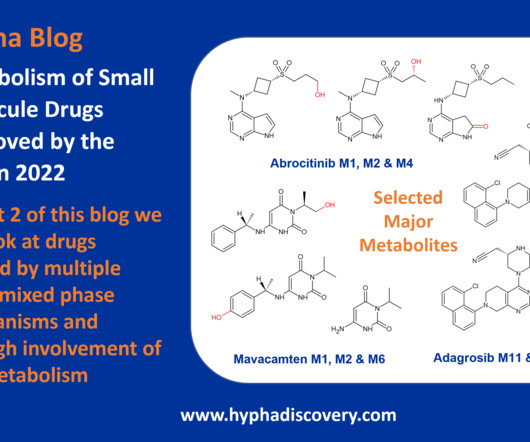
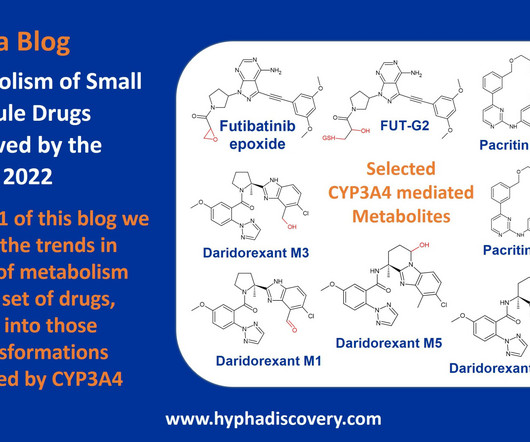
Metabolism of 2023 FDA Approved Small Molecules – PART 1 By Julia Shanu-Wilson 2023 was a fruitful year for drug approvals by the FDA, with a crop of 34 small molecules out of a total of 55 new drugs [1]. are major metabolites according the FDA Metabolites in Safety Testing guidelines).












































Let's personalize your content