Novavax Announces COVID-19 Vaccine Clinical Development Progress
The Pharma Data
NOVEMBER 30, 2020
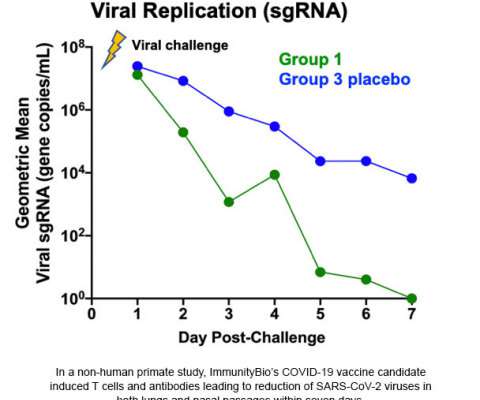
government to meet its Operation Warp Speed goals to expedite the delivery of millions of doses of safe, effective vaccines for COVID-19. About NVX-CoV2373 NVX-CoV2373 is a protein-based vaccine candidate engineered from the genetic sequence of SARS-CoV-2, the virus that causes COVID-19 disease. Novavax was awarded $1.6


























Let's personalize your content