4-1BB and the critical importance of local control
SugarCone Biotech
JUNE 10, 2024
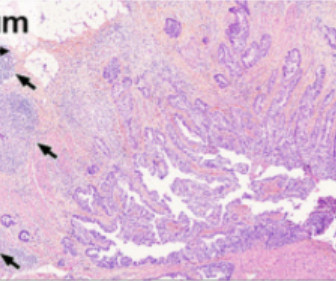
[link] Immunity is complex and can be dangerous when exploited clinically, as demonstrated by the lethal administration of TNF, or anti-CD40L antibody (Biogen) or CAR-T cells expressing the CD16 Fc-receptor (Unum), among many other examples. This is the T cell type most closely associated with anti-tumor immune responses.




































Let's personalize your content