Precision for Medicine Receives CAP-Accreditation for Full-Service Histopathology Laboratory
Precision for Medicine
DECEMBER 1, 2023
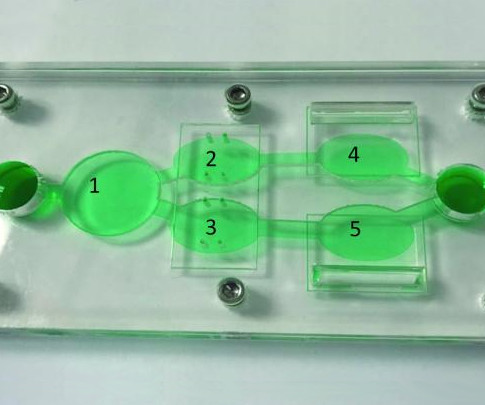
December 1, 2023 — Precision for Medicine, the first global biomarker-driven clinical research organization, is the recipient of a new accreditation from The College of American Pathologists (CAP) for its tissue and histopathology laboratory in Winston-Salem, North Carolina. Winston-Salem, Nc.,









































Let's personalize your content