Study proves efficacy of novel protein conjugate in autoimmune disease
Drug Discovery World
JUNE 10, 2024
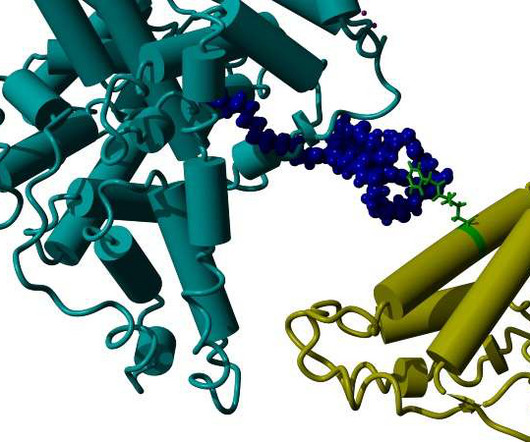
VALANX is researching and developing protein expression systems that allow its own synthetic amino acids to be incorporated into any protein at freely selectable positions in multiple copies.


























Let's personalize your content