Hybrid FSP/FSO Solutions Enable Flexibility and Agility to Keep Biotech Trials On Time and On Budget
PPD
OCTOBER 8, 2024
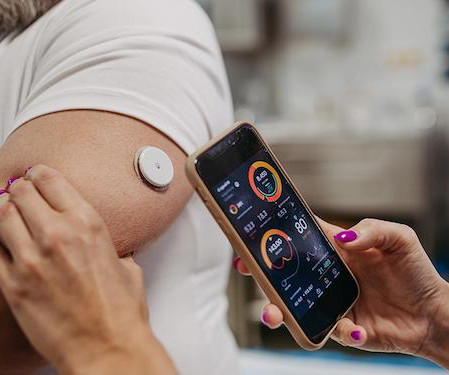
The highly dynamic biotech industry has a core need to remain as flexible and agile as possible across the spectrum of clinical development activities. One primary way these companies create a nimble and adaptive environment is by outsourcing some portion of clinical development functions.

















Let's personalize your content