Webinar: Assessing Cognition Impairment Using Driving Simulators in Clinical Trials
Alta Sciences
FEBRUARY 8, 2024
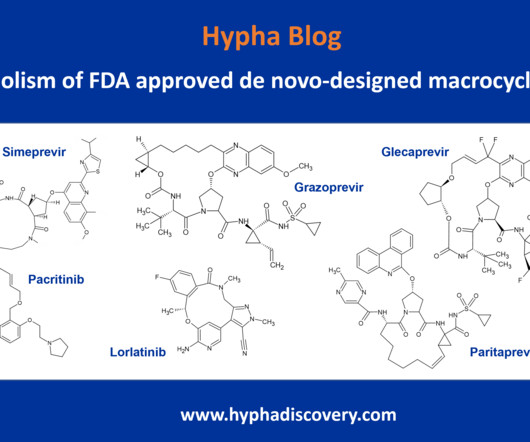
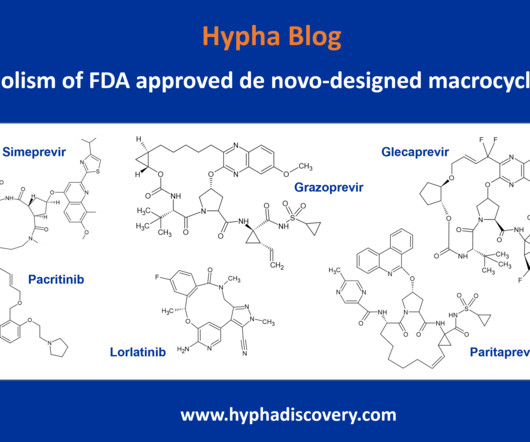
Utilizing 10 state-of-the-art simulators available in-house at our Montréal clinical facility (with the capacity for more than 20), we are equipped to measure a range of studies; from impairment in cognition and comparing compounds to assessing the impact on new formulations have on impairment.





















Let's personalize your content