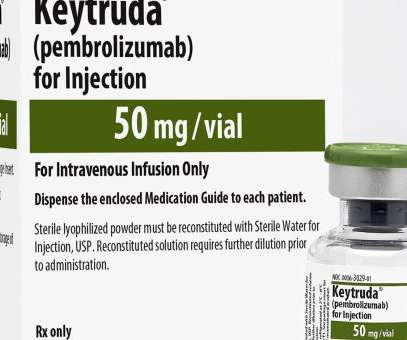
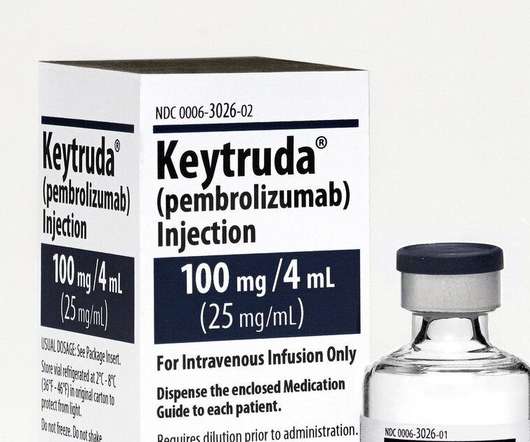
FDA Approves Updated Indication for Merck’s KEYTRUDA® (pembrolizumab) for Treatment.
The Pharma Data
AUGUST 31, 2021
10), as determined by an FDA-approved test, or in patients who were not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. This indication was approved under accelerated approval based on tumor response rate and duration of response.





















Let's personalize your content