Utilising engineered peptides for immunotherapy
Drug Target Review
MAY 14, 2024
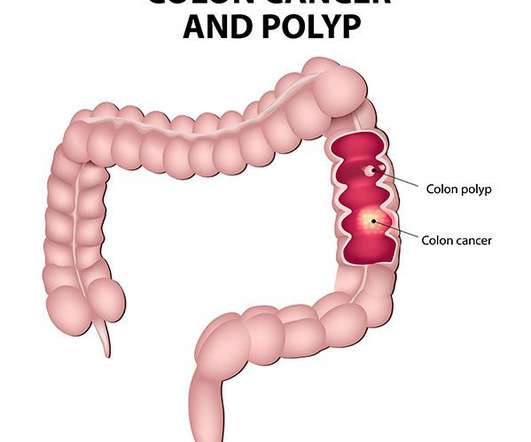
The combination of P1 with antiPD1 more efficiently activated innate and adaptive immune responses than either P1 or antiPD1 alone by re-energising T cell effector functions, thus providing the remarkable reduction of tumour growth in solid tumours and extended survival period.









































Let's personalize your content