Current insights and molecular docking studies of HIV?1 reverse transcriptase inhibitors
Chemical Biology and Drug Design
OCTOBER 10, 2023
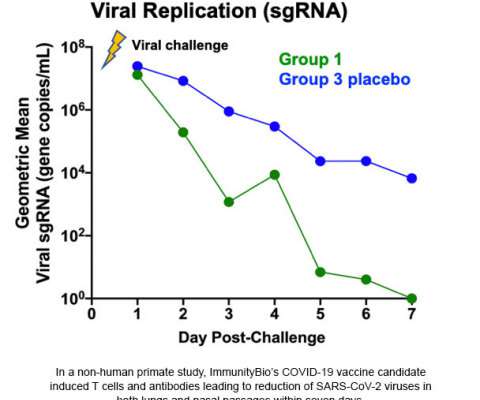
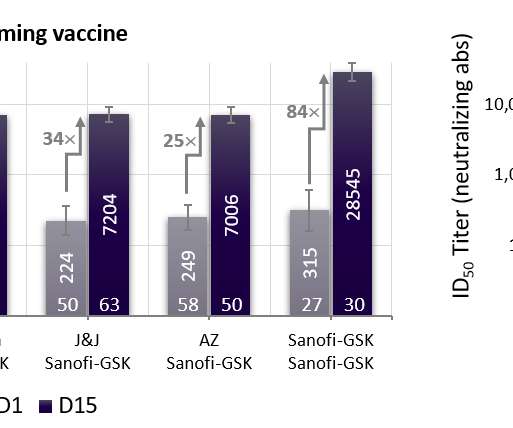
Abstract Human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome (AIDS), a lethal disease that is prevalent worldwide. Later, a new type of non-nucleoside reverse transcriptase inhibitors (NNRTIs) were approved as anti-HIV drugs. Molecular insights of HIV Reverse transcriptase and it's inhibitors.












































Let's personalize your content