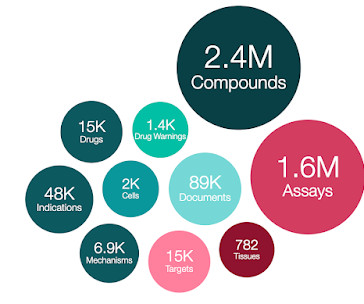
ChEMBL 34 is out!
The ChEMBL-og
APRIL 16, 2024
71 out of the 882 newly added EMA drugs are only authorised by EMA, rather than from other regulatory bodies e.g. FDA. The definition of a chemical probe has been amended to update the field MOLECULE_DICTIONARY.CHEMICAL_PROBE. University of Dundee: T. 1813 bioactivities in total have been added.

















Let's personalize your content