How the Classic TB Vaccine Treats Bladder Cancer – Zebrafish Avatars Reveal Mechanism
PLOS: DNA Science
OCTOBER 3, 2024
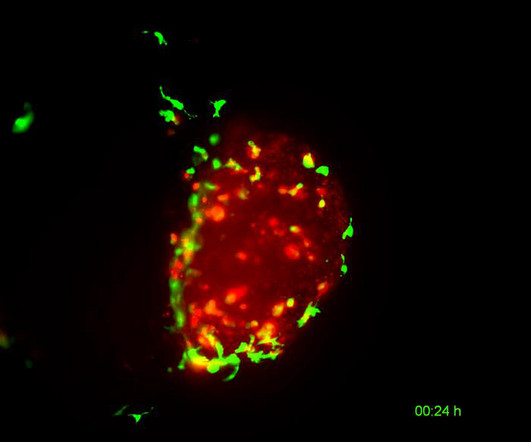
One of the oldest and most successful immunotherapies is simpler: a tamed version of a classic vaccine, against the infectious disease tuberculosis (TB). “BCG” is the “treatment” vaccine’s technical name, for Mycobacterium bovis Bacillus Calmette-Guérin. The approach worked.


























Let's personalize your content