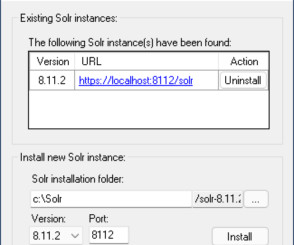
Still working with Sitecore without Docker? Check Sifon, your new multitool in a Sitecore toolbelt
Perficient: Drug Development
SEPTEMBER 25, 2023
Hey Sitecore professionals, if you have not heard about Sifon for Sitecore – you must definitely check this out. I spent a lot of time building and testing it and can say Sifon is a definite Swiss army knife for local Sitecore development in the right arms, so you’d really like to learn why. Just one click to it.


































Let's personalize your content