Drug development practices to improve public health policy
Drug Discovery World
DECEMBER 6, 2022
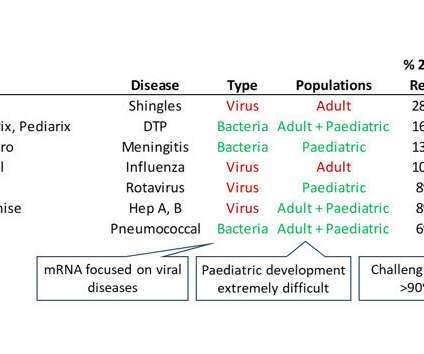
As a result of clear US Food and Drug Administration (FDA) guidelines, the leading vaccine development programmes for Covid-19 were all remarkably similar. As a result of clear US Food and Drug Administration (FDA) guidelines, the leading vaccine development programmes for Covid-19 were all remarkably similar.






























Let's personalize your content