COVID Hospitalizations in U.S. Hit Record High
The Pharma Data
DECEMBER 1, 2020
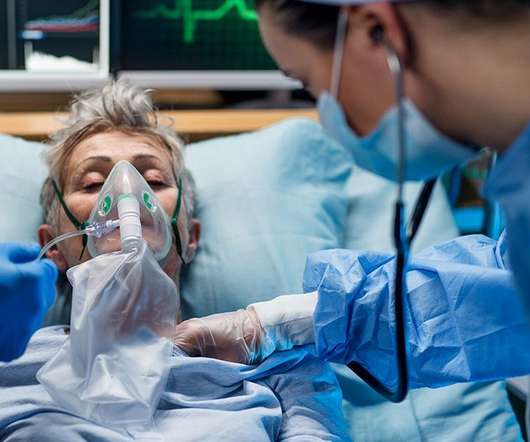
1, 2020 — Yet another grim record for COVID-19 hospitalizations was set on Monday, as more than 96,000 patients battled severe cases of the virus in hospital wards across America. But the number of hospitalizations have risen by more than 12 percent over the past week, the newspaper noted. TUESDAY, Dec. California Gov.





































Let's personalize your content