ADCs: the next generation of targeted therapies
Drug Discovery World
SEPTEMBER 25, 2024
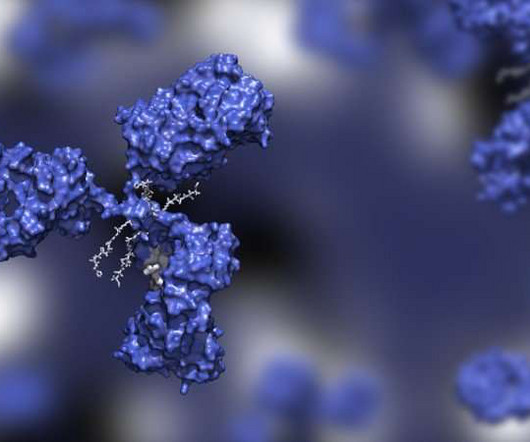
Mylotarg was ahead of its time in many ways: its novel structure of an antibody to target diseased cells, plus a cancer-killing payload attached via a special linker, is the blueprint for the antibody-drug conjugates (ADCs) that followed.














































Let's personalize your content