Pfizer wins FDA approval of new meningococcal vaccine
BioPharma Drive: Drug Pricing
OCTOBER 23, 2023
The clearance of the pentavalent shot Penbraya adds to Pfizer’s infectious disease portfolio as it adjusts to slumping COVID-19 vaccine sales.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

BioPharma Drive: Drug Pricing
OCTOBER 23, 2023
The clearance of the pentavalent shot Penbraya adds to Pfizer’s infectious disease portfolio as it adjusts to slumping COVID-19 vaccine sales.

The Pharma Data
AUGUST 15, 2021
Food and Drug Administration (FDA) has approved TICOVAC (tick-borne encephalitis (TBE) vaccine) for active immunization to prevent TBE in individuals 1 year of age and older. 1 TICOVAC is the only FDA-approved vaccine to help protect U.S. Following today’s FDA approval, the U.S. 45 years ago.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The Pharma Data
APRIL 26, 2022
Before now, Veklury was only approved to treat certain adults and pediatric patients (12 years of age and older who weigh at least 40 kilograms, which is about 88 pounds) with COVID-19. “As director of the FDA’s Center for Drug Evaluation and Research. The FDA urges the public to get vaccinated and receive a booster when eligible.

The Pharma Data
NOVEMBER 12, 2021
Treatment is First FDA-Approved Option Patients Can Take Regardless of Previous Therapies. Food and Drug Administration approved Besremi (ropeginterferon alfa-2b-njft) injection to treat adults with polycythemia vera, a blood disease that causes the overproduction of red blood cells. The FDA, an agency within theU.S.

The Pharma Data
JUNE 18, 2021
First approval of a conjugate vaccine that helps protect against 20 serotypes responsible for the majority of invasive pneumococcal disease and pneumonia, 1,2,3,4,5,6,7 including seven responsible for 40% of pneumococcal disease cases and deaths in the U.S. Following today’s FDA approval, the U.S.

The Pharma Data
JULY 29, 2021
Food & Drug Administration (FDA) has extended the shelf life for the Johnson & Johnson single-shot COVID-19 vaccine to six months. Expiration dates will be updated on www.vaxcheck.jnj , where vaccine providers can confirm the latest expiration dates of our vaccine. We continue to work with the U.S.

The Pharma Data
MAY 26, 2023
Pfizer’s PAXLOVID™ receives FDA approval for adult patients at high risk of progression to severe COVID-19 Pfizer Inc. These real-world studies also have shown that PAXLOVID is effective amongst both vaccinated and unvaccinated high-risk patients.(6,7,8,9,10) NYSE: PFE) announced today that the U.S. Source link: [link]

The Pharma Data
MAY 6, 2022
Food and Drug Administration (FDA) to expand the approval of COMIRNATY® (COVID-19 Vaccine, mRNA) to include individuals ages 12 through 15 years. In the trial, a two-dose series of the Pfizer-BioNTech COVID-19 Vaccine (30-µg per dose) was 100% effective (95% confidence interval [CI, 87.5,

The Pharma Data
JANUARY 17, 2021
plans to create a global R&D achievement based on innovations of inflammation–fibrosis treatment, Triple-acting new drug for NASH (non-alcoholic steatohepatitis) treatment as well as various other innovations in metabolic disease, oncology and rare disease fields. are expected to be approved by the U.S.

The Pharma Data
NOVEMBER 29, 2021
The American Cancer Society estimates there will be more than 21,000 new cases of ovarian cancer and more than 13,000 deaths from this disease in 2021, making it the deadliest of all female reproductive system cancers. The FDA, an agency within the U.S.
The Pharma Data
FEBRUARY 23, 2022
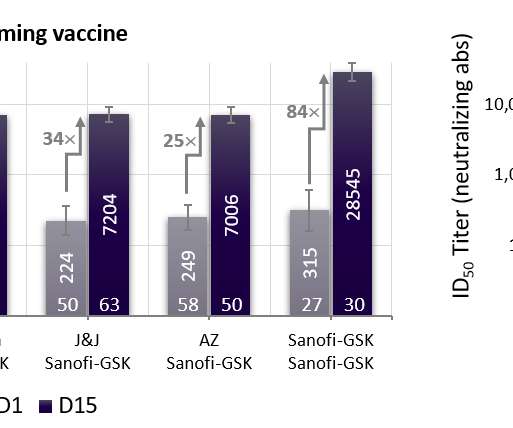
* Final analysis of the global VAT02 booster trial confirms universal ability to boost neutralizing antibodies 18- to 30-fold across vaccine platforms (mRNA, adenovirus). * efficacy against any symptomatic COVID-19 disease, in line with expected vaccine effectiveness in today’s environment dominated by variants of concern.
The Pharma Data
JULY 12, 2021
Rare cases of the neurological disorder, Guillain-Barré syndrome have been reported following vaccination with the Janssen COVID-19 vaccine. Most occurred within 42 days after vaccination. For further information on the safety of authorized COVID-19 vaccines, please visit: [link]. What Is the Janssen COVID-19 Vaccine?

The Pharma Data
MARCH 29, 2022
Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series.

The Pharma Data
NOVEMBER 18, 2020
” COVID Vaccines Move Closer to Emergency Use Approval. Pfizer and the German firm BioNTech announced Thursday that their vaccine is 95 percent effective and they will apply for emergency use approval “within days.” announced earlier this week that early results show its coronavirus vaccine is 94.5

The Pharma Data
NOVEMBER 16, 2020
November 16, 2020 — An independent data and safety monitoring board (DSMB) overseeing the Phase 3 trial of the investigational COVID-19 vaccine known as mRNA-1273 reviewed trial data and shared its interim analysis with the trial oversight group on Nov. The interim analysis comprised 95 cases of symptomatic COVID-19 among volunteers.

The Pharma Data
NOVEMBER 21, 2021
“Today’s approval fulfills an unmet medical need for more than 10,000 children in the United States and underscores the FDA’s commitment to help make new therapies available for rare diseases,” said Theresa Kehoe, M.D., director of the Division of General Endocrinology in the FDA’s Center for Drug Evaluation and Research.

The Pharma Data
DECEMBER 16, 2020
Food and Drug Administration (FDA) advisory panel in regard to the COVID-19 vaccine from Moderna. The Vaccines and Related Biological Products Advisory Committee will be determining whether the product should be authorized for emergency use, according to CNBC. The vote itself is not slated to take place until after 3 p.m.

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been recommended for conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been granted a conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.
The Pharma Data
MARCH 2, 2021
Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for its single-dose COVID-19 vaccine, developed by the Janssen Pharmaceutical Companies of Johnson & Johnson, to prevent COVID-19 in individuals 18 years of age and older. The terms of the EUA allow use of the vaccine while more data are gathered.

Codon
NOVEMBER 3, 2024
Physicians working in the early 20th century had little choice but to treat the world’s most rampant infectious disease with methods such as these. Even after microbiologists discovered the bacterium that causes the illness in 1882, it wasn’t until the 1920s that researchers were able to develop a vaccine for TB.

The Pharma Data
FEBRUARY 24, 2022
Jardiance was originally approved by the FDA in 2014 as a supplement to diet and exercise to improve glucose control in adults with type 2 diabetes. Today’s approval will provide a treatment option for a wider range of patients with heart failure,” said Norman Stockbridge, M.D., The FDA, an agency within the U.S.

The Pharma Data
JANUARY 15, 2021
Food and Drug Administration (FDA) approval of Darzalex Faspro ® (daratumumab and hyaluronidase-fihj), a subcutaneous formulation of daratumumab, in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd) for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis.[1]

The Pharma Data
NOVEMBER 23, 2020
. Positive high-level results from an interim analysis of clinical trials of AZD1222 in the UK and Brazil showed the vaccine was highly effective in preventing COVID-19, the primary endpoint, and no hospitalisations or severe cases of the disease were reported in participants receiving the vaccine.

The Pharma Data
NOVEMBER 30, 2020
Nasdaq: NVAX), a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, today provided an update on its COVID-19 vaccine program. Vaccines Taskforce and National Institute for Health Research played pivotal roles in the rapid recruitment and enrollment of volunteers.

The Pharma Data
MARCH 15, 2022
COPD, which includes emphysema and chronic bronchitis, is a long-term, chronic disease that causes airflow blockage and makes it difficult to breathe. The inhaler is approved for two strengths (160/4.5 The FDA, an agency within the U.S. It can cause wheezing (a whistling sound when breathing), shortness of breath, and coughing.

The Pharma Data
DECEMBER 30, 2020
30 December 2020 — AstraZeneca’s COVID-19 vaccine has been approved for emergency supply in the UK, with the first doses being released today so that vaccinations may begin early in the New Year. This is the first authorisation for this vaccine.

The Pharma Data
FEBRUARY 24, 2022
If the EC grants the variation, the decision will be immediately applicable to all 27 EU member states, making booster vaccines available to everyone 12 years and older. . The COVID-19 vaccine booster for individuals 12 through 15 years of age was already granted Emergency Use Authorization by the U.S. and Europe.

The Pharma Data
NOVEMBER 9, 2020
Nasdaq: NVAX), a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for NVX-CoV2373, the Company’s COVID-19 vaccine candidate. GAITHERSBURG, Md., Glenn, M.D., government.
The Pharma Data
DECEMBER 11, 2020
Food and Drug Administration is expected to approve emergency use of Pfizer’s coronavirus vaccine as early as Saturday after its advisory panel cleared the way for the start of a national campaign to inoculate Americans and stem the spread of COVID-19. Centers for Disease Control and Prevention. Who is first in line?

The Pharma Data
DECEMBER 18, 2020
Food and Drug Administration issued an emergency use authorization (EUA) for the second vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The emergency use authorization allows the Moderna COVID-19 Vaccine to be distributed in the U.S.

The Pharma Data
JANUARY 31, 2021
Adjuvanted S-Trimer COVID-19 vaccine candidates demonstrated favorable safety and tolerability profiles and strong neutralizing immune responses in a phase 1 trial. Clover plans to initiate a global phase 2/3 trial in the first half of 2021 with an interim analysis for vaccine efficacy potentially in the middle of 2021.

The Pharma Data
DECEMBER 3, 2020
The Phase 1 trial was a randomized, observer-blind, placebo-controlled study to assess the safety, reactogenicity and immunogenicity of the adjuvanted COVID-19 S-Trimer vaccine candidates formulated with different antigen levels. No serious adverse events related to the vaccine candidates studied were reported.

The Pharma Data
JANUARY 6, 2021
6 January 2021 — AstraZeneca’s COVID-19 vaccine has been granted emergency use authorisation in India as well as Argentina, Dominican Republic, El Salvador, Mexico and Morocco for the active immunisation of adults. Additional safety and efficacy data will continue to accumulate from ongoing clinical trials.
DrugBank
MAY 6, 2024
This category encompasses a broad spectrum of treatments including vaccines, blood components, gene therapies, and more, each tailored to combat diseases in highly specific ways. A Wave of Innovations The year 2024 has already proven to be significant for the field of biologics, witnessing a notable increase in FDA approvals.

The Pharma Data
AUGUST 20, 2020
The Phase III study is evaluating the safety, tolerability and immunogenicity of GSK’s MenABCWY vaccine candidate compared to Bexsero and Menveo. Invasive Meningococcal Disease (IMD) is uncommon, with country-specific reported cases ranging from 0.1 cases per 100,000 population in 2017. Source link.

The Pharma Data
NOVEMBER 23, 2021
director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”. The FDA, an agency within theU.S.

Vial
DECEMBER 11, 2023
Pathophysiological evidence links HCV infections to the risk of liver diseases such as hepatocellular carcinoma (HCC) and liver cirrhosis. The Food and Drug Administration (FDA) approved and recommended dozens of small-molecule drugs. Patients at advanced stages of liver disease can still be treated.

The Pharma Data
OCTOBER 9, 2021
Application utilizes extrapolation-based strategy across existing breadth of STELARA data in patients living with this chronic inflammatory disease. Food and Drug Administration (FDA) seeking expanded approval of STELARA® (ustekinumab) to treat pediatric patients ages 5 years and older with juvenile psoriatic arthritis (jPsA).

The Pharma Data
AUGUST 14, 2020
In other news, Genentech’s Evrysdi (risdiplam) has secured FDA approval for the treatment of spinal muscular atrophy and Bristol Myers Squibb has announced positive data from two Phase 3 clinical trials of its drug Opdivo. AstraZeneca seals manufacturing deal to deliver COVID-19 vaccine in China.

The Pharma Data
FEBRUARY 23, 2022
Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted to recommend Pfizer’s TicoVac TM (tick-borne encephalitis (TBE) vaccine) for active immunization to prevent TBE in individuals 1 year of age and older, in the following U.S. Global President, Vaccines, Pfizer. “We

The Pharma Data
AUGUST 28, 2020
The top 10 news stories this week are focused on coronavirus vaccines and treatments, as China is set to move ahead with human testing of a potential vaccine that has been created using insect cells, while AstraZeneca has started a clinical trial of its antibody treatment designed to prevent and also treat symptoms of coronavirus.

The Pharma Data
DECEMBER 31, 2020
Moderna and Pfizer’s COVID-19 Vaccines Roll Out in Early Phase. Food and Drug Administration (FDA) issued Emergency Use Authorization for two COVID-19 vaccines: Pfizer-BioNTech’s BNT162b2 and Moderna’s mRNA-1273. In studies, the vaccines were found to be 94% to 95% effective in preventing COVID-19 disease.

The Pharma Data
NOVEMBER 16, 2020
We believe that our investments in mRNA delivery technology and manufacturing process development will allow us to store and ship our COVID-19 vaccine candidate at temperatures commonly found in readily available pharmaceutical freezers and refrigerators,” said Juan Andres, Chief Technical Operations and Quality Officer at Moderna. “We
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content