FDA Advisors Say New Gene Therapy for Sickle Cell Disease, Exa-cel, is Safe
Drugs.com
NOVEMBER 2, 2023
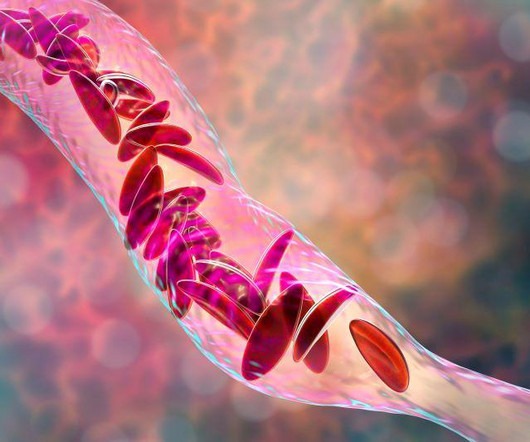
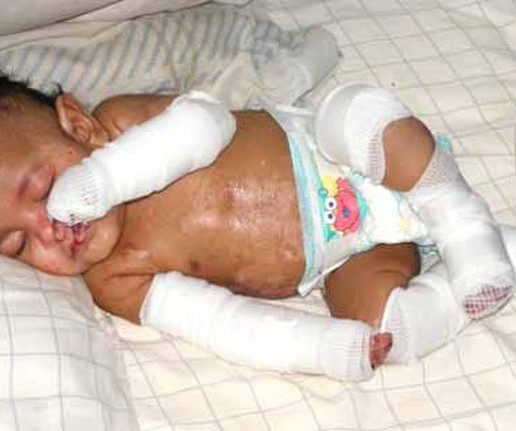
1, 2023 -- A new gene therapy for sickle cell disease was deemed safe by a U.S. The FDA had already decided that the. WEDNESDAY, Nov. Food and Drug Administration advisory panel on Tuesday, paving the way for full approval by early December.




































Let's personalize your content