Health Care Workers, Nursing Home Residents to Get First Vaccines: Panel
The Pharma Data
DECEMBER 1, 2020
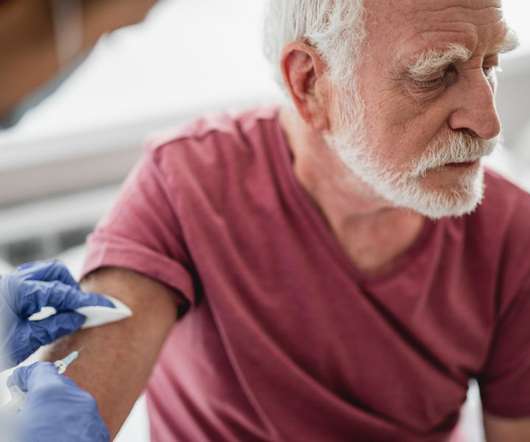
1, 2020 — Health care workers and people in nursing homes should be at the front of the line for upcoming COVID-19 vaccines, a U.S. Centers for Disease Control and Prevention advisory panel recommended Tuesday. In 2021, five to 10 million doses of vaccine are anticipated to ship each week. TUESDAY, Dec.





























Let's personalize your content