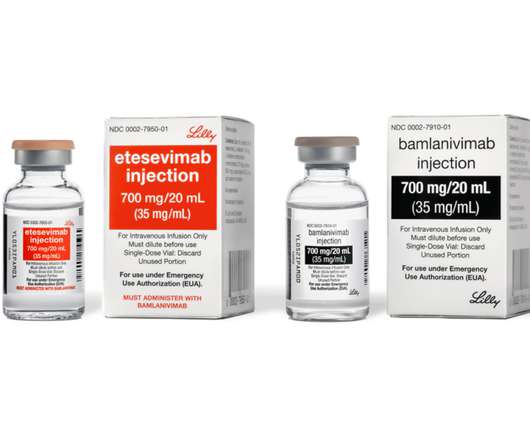
bamlanivimab and etesevimab together for treatment of COVID-19 in the U.S.
The Pharma Data
APRIL 16, 2021
1.429 California strain that currently accounts for 50 percent of the virus in California and over 10 percent across a number of additional states. However, sites of care should not dispose of bamlanivimab supply; instead, they should order etesevimab to pair with it. All sites in the U.S. In the U.S.,












Let's personalize your content