A therapy candidate for fatal prion diseases turns off disease-causing gene
Broad Institute
JUNE 27, 2024
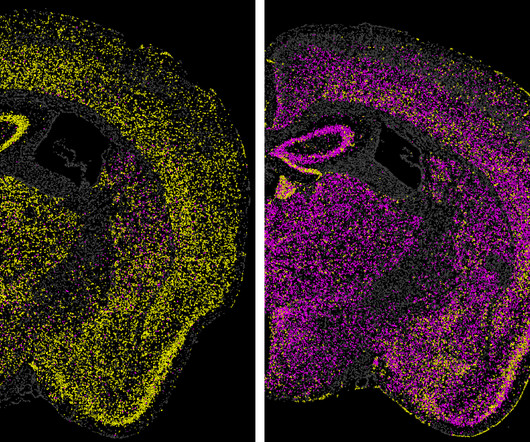
Science (2024) Related content New gene delivery vehicle shows promise for human brain gene therapy My Quest to Cure Prion Disease — Before It’s Too Late | Sonia Vallabh | TED Prion diseases lead to rapid neurodegeneration and death and are caused by misshapen versions of the prion protein in the brain.



































Let's personalize your content