Huntington’s disease and potential therapies
Drug Target Review
AUGUST 22, 2023
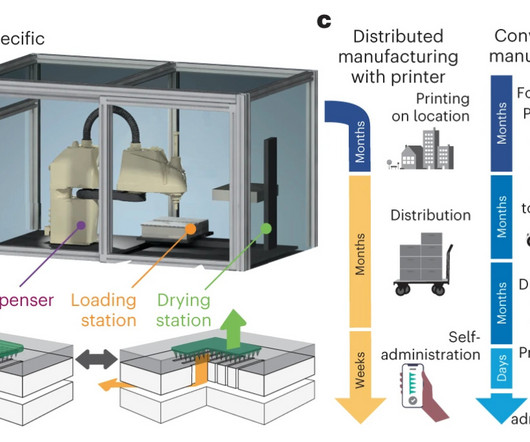
This Q&A explores how pre-clinical research is being used to identify potential therapies for Huntington’s disease, a devastating condition that currently lacks disease- modifying treatments. What makes the potential therapeutic for Huntington’s disease unique compared to other treatments?







































Let's personalize your content