Moderna, Merck advance cancer vaccine into late-stage test
BioPharma Drive: Drug Pricing
JULY 26, 2023
The companies are enrolling people with melanoma in a Phase 3 trial aimed at testing whether the shot can prevent disease recurrence.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
BioPharma Drive: Drug Pricing
JULY 26, 2023
The companies are enrolling people with melanoma in a Phase 3 trial aimed at testing whether the shot can prevent disease recurrence.

PPD
JUNE 24, 2024
In the realm of vaccine development, mega trials — studies enrolling 5,000 subjects or more — have been instrumental in the fight against many pathogens, including influenza, rotavirus, malaria, RSV and most recently in the rapid development of vaccines against COVID-19.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Codon
NOVEMBER 3, 2024
Physicians working in the early 20th century had little choice but to treat the world’s most rampant infectious disease with methods such as these. Even after microbiologists discovered the bacterium that causes the illness in 1882, it wasn’t until the 1920s that researchers were able to develop a vaccine for TB.

BioPharma Drive: Drug Pricing
OCTOBER 7, 2024
The development of cancer vaccines has provided some hope in the battle against cancer worldwide; however, there are still many challenges to overcome when developing these life-saving treatments.

DrugBank
MARCH 12, 2025
The COVID-19 pandemic brought mRNA to global prominence by developing highly effective vaccines by Moderna and Pfizer-BioNTech. However, mRNA technology is not limited to infectious diseases. However, mRNA technology has introduced new possibilities, particularly in developing personalized cancer vaccines and combination therapies.

BioPharma Drive: Drug Pricing
OCTOBER 12, 2023
Earlier this year Pfizer removed thousands of participants from a study of its Lyme disease shot over concerns the group, Care Access, wasn't meeting clinical practice standards.

DrugBank
JULY 18, 2024
Vaccines have consistently demonstrated their efficacy in protecting people from infectious diseases. This led to the eradication of smallpox and polio – two debilitating diseases that historically caused global epidemics. Sugars, such as sucrose are common stabilizers in many vaccines.

DrugBaron
JANUARY 2, 2021
Over the past week a furious debate has broken out over the merits of delaying the second dose of the approved COVID vaccines, to increase the number of people who can be given a single dose. The case against doing so is clear: the labels of the approved vaccines are unambiguous that two doses should be given within a certain time window.

The Pharma Data
JULY 21, 2021
Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, and Pfizer Inc. If successful, this trial could enable the inclusion of a pediatric population in the Phase 3 trial.

Codon
JANUARY 21, 2024
Today, refined versions of these human challenge studies have become standard practice in testing vaccines for vector-borne diseases (e.g., yellow fever, malaria, and dengue), evaluating new drugs or treatments, and studying pathogenesis, the process by which a disease develops. Why are challenge trials becoming more popular?

SCIENMAG: Medicine & Health
JUNE 12, 2023
Peer-reviewed / Randomised Controlled Trial / People Study of healthy US adults found that a single dose of the VLA1553 vaccine candidate was generally safe, well tolerated and provokes an immune response.
SCIENMAG: Medicine & Health
NOVEMBER 27, 2023
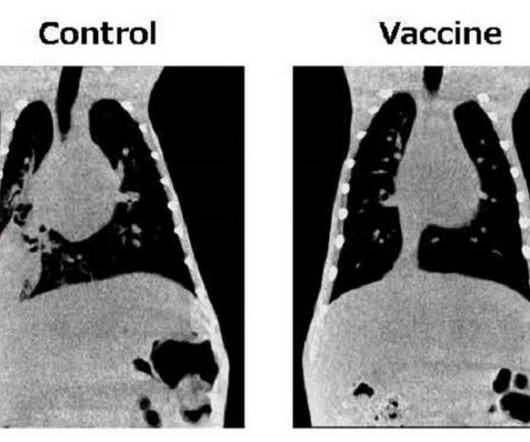
Osaka, Japan – The global impact of the coronavirus pandemic has ignited a renewed focus on emerging and re-emerging infectious diseases. Researchers at Osaka Metropolitan University are making great strides in combating pneumococcal pneumonia, one of the leading causes of respiratory deaths worldwide.
SCIENMAG: Medicine & Health
SEPTEMBER 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland.

The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. The trial will evaluate the safety and immunogenicity of a monovalent flu vaccine candidate coding for the hemagglutinin protein of the A/H3N2 strain of the influenza virus. JUNE 22 , 2021.

The Pharma Data
APRIL 26, 2022
Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company, and Pfizer Inc. Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company, and Pfizer Inc. NYSE: PFE) today reported positive Phase 2 pediatric data for their Lyme disease vaccine candidate, VLA15.
The Pharma Data
JUNE 25, 2021
In the unprecedented context of at least 13 variants circulating within the study population subset assessed at this interim analysis, CVnCoV demonstrated an interim vaccine efficacy of 47% against COVID-19 disease of any severity and did not meet prespecified statistical success criteria. About CVnCoV.

PPD
MAY 11, 2023
The global COVID-19 pandemic increased awareness of the importance of vaccine development — both for drug developers and the public. The speed at which COVID-19 vaccines were developed was remarkable, but like most newly developed vaccines, there was variation among who could receive the shots and when.

FDA Law Blog: Drug Discovery
JUNE 12, 2024
Sasinowski — Hyman, Phelps & McNamara (HPM) would like to congratulate the 7 rare disease programs selected for the inaugural class of the FDA’s “Support for clinical Trials Advancing Rare disease Treatment” (START) pilot program.
Drug Target Review
JANUARY 23, 2024
Vaccinations against tumour antigens that are shared between tumours, or tumour antigens that arise from mutations unique to individual tumours, represent promising strategies. Cell-based therapies, such as tumour-infiltrating lymphocyte (TIL) therapy, have also drawn significant attention following promising results in a melanoma trial.
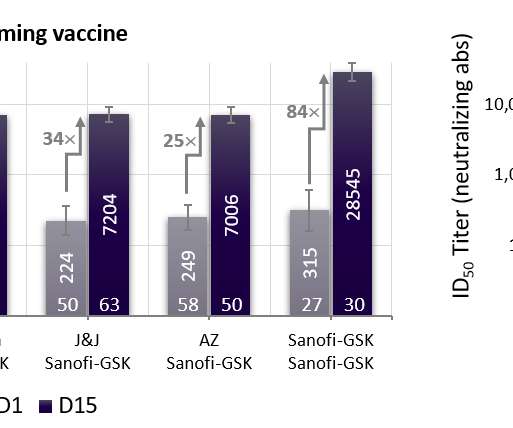
The Pharma Data
FEBRUARY 23, 2022
* Final analysis of the global VAT02 booster trial confirms universal ability to boost neutralizing antibodies 18- to 30-fold across vaccine platforms (mRNA, adenovirus). * efficacy against any symptomatic COVID-19 disease, in line with expected vaccine effectiveness in today’s environment dominated by variants of concern.

The Pharma Data
JUNE 24, 2021
The primary objective in the trial is to describe safety when both vaccines are co-administered, with follow up six months after vaccination. Secondary objectives are to describe immune responses produced by each of the vaccines. Secondary objectives are to describe immune responses produced by each of the vaccines.

The Pharma Data
MAY 16, 2021
Adjuvanted recombinant COVID-19 vaccine candidate triggered strong neutralizing antibody responses in all adult age groups. Overall, the vaccine candidate elicited strong neutralizing antibody levels that were comparable to those generated by natural infection, with higher levels observed in younger adults (18 to 59 years old).

The Pharma Data
MARCH 31, 2022
A Phase 2 clinical trial evaluating various additional COVID-19 booster shots has begun enrolling adult participants in the United States. The study, known as the COVID-19 Variant Immunologic Landscape (COVAIL) trial, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
The Pharma Data
SEPTEMBER 2, 2021
First subjects vaccinated in study of Pfizer’s RSV bivalent prefusion F subunit investigational vaccine candidate in adults ages 60 or older. RSV is a common and pervasive cause of severe acute respiratory illness in older adults with no vaccine currently available. NEW YORK–(BUSINESS WIRE)– Pfizer Inc. Jansen, Ph.D.,

H1 Blog
SEPTEMBER 26, 2023
Breaking Down Barriers to International Clinical Trials Global Disruptions, Health Equity & Data Sharing As the U.S. When it comes to developing new treatments and vaccines, the development timeline is often long and arduous, especially outside of the U.S. Additionally, how do you ensure that the vaccine distribution is equitable?

PLOS: DNA Science
JULY 27, 2023
That information led, thanks to vaccine shelved from the first SARS circa 2003, to the rapid development and deployment of mRNA vaccines against the new infectious disease. Next up: testing in real-world settings and submission of clinical trial findings to the FDA.

The Pharma Data
OCTOBER 14, 2021
Biogen today announced results of a new analysis of immune response to the COVID-19 vaccine among people with multiple sclerosis (MS). Germany and Spain, researchers evaluated blood samples from 322 participants 28-90 days after their last COVID-19 vaccine dose. Using data from the MS PATHS network in the U.S.,

Alta Sciences
SEPTEMBER 27, 2024
Featuring two scenarios that explore the complexities of bioanalysis for immunomodulators, The Altascientist offers practical considerations for ensuring accurate bioanalysis, as well as pharmacokinetic, pharmacodynamic , and safety data in clinical trials. Each class of immunomodulator has a defined complexity and mechanism of action.

The Pharma Data
JUNE 17, 2022
In a Phase 2/3 clinical trial, the Pfizer-BioNTech COVID-19 Vaccine elicited a strong immune response in this age group Three 3-µg doses had favorable safety profile similar to placebo in young children ages 6 months through 4 years in Phase 2/3 clinical trial Pfizer-BioNTech COVID-19 Vaccine now authorized in the U.S.

The Pharma Data
JUNE 24, 2021
This follows the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) positive opinion to authorize the vaccine in this age group. COMIRNATY was the first COVID-19 vaccine to receive authorization in the EU and is the first to have its CMA extended to adolescents. g doses of the COVID-19 vaccine.

The Pharma Data
AUGUST 22, 2021
The trial accrued 25 cases of symptomatic COVID-19 at the primary analysis.There were no cases of severe COVID-19 or COVID-19-related deaths in those treated with AZD7442. The trial included 5,197 participants in a 2:1 randomisation AZD7442 to placebo. The trial was conducted in 87 sites in the US, UK, Spain, France and Belgium.
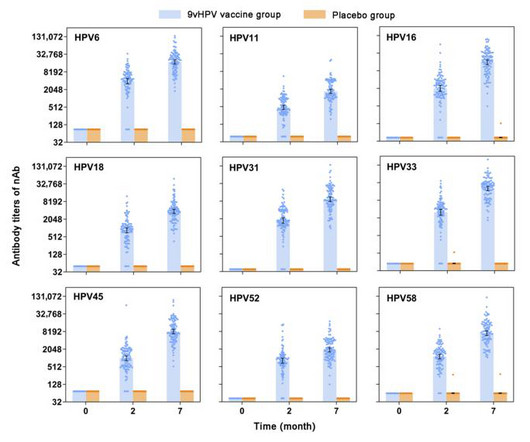
SCIENMAG: Medicine & Health
OCTOBER 18, 2023
This study is conducted by the Jiangsu Provincial Center for Disease Control and Prevention, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Vaccines for Infectious Diseases of Xiamen University, China National Institutes for Food and Drug Control, and Xiamen Innovax Biotech Company.
The Pharma Data
AUGUST 3, 2021
Accelerates development of current Sanofi licensed programs in vaccines and potential to explore other therapeutic areas Fast tracks establishment of Sanofi’s recently announced mRNA Center of Excellence Full integration upgrades drug formulation capabilities and enhances US talent in a promising new technology.
The Pharma Data
SEPTEMBER 28, 2021
Many parents have questions on COVID-19 and when vaccines are going to be available for youngsters younger than 12 years aged. We are therefore also wanting to see COVID-19 vaccines available for young children. Some have stated that they’re still enrolling, and a few are still administering doses or following participants.

Broad Institute
AUGUST 7, 2024
Anna Greka ’s research focuses on fundamental mechanisms of cellular dysfunction in genetic diseases with a focus on membrane proteins. Some of her discoveries have already led to clinical trials. She also leads Broad’s rare disease initiative, Ladders to Cures. She is a professor at the Harvard T.H.
The Pharma Data
SEPTEMBER 30, 2021
An expert review by a world group of scientists, including some at the WHO and FDA, concludes that, even for the delta variant, vaccine efficacy against severe COVID is so high that booster doses for the overall population aren’t appropriate at this stage within the pandemic.
The Pharma Data
JULY 18, 2021
VAXNEUVANCE Elicited Superior Immune Responses for Serotypes 3, 22F and 33F Compared to PCV13, Which Are Major Causes of Disease. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) is expected to meet in October to discuss and make recommendations on the use of VAXNEUVANCE in adults.

Codon
AUGUST 26, 2024
He also explains how they could be used to combat pandemics on “day zero,” well before vaccines are developed. After the outbreak ended, it took another three years for the first Ebola vaccine by Merck to be approved. Timelines for vaccine development are shrinking, but can it move even faster?

The Pharma Data
AUGUST 15, 2021
Food and Drug Administration (FDA) has approved TICOVAC (tick-borne encephalitis (TBE) vaccine) for active immunization to prevent TBE in individuals 1 year of age and older. 1 TICOVAC is the only FDA-approved vaccine to help protect U.S. We are proud to deliver the first vaccine to help protect people in the U.S. 45 years ago.

The Pharma Data
JUNE 18, 2021
First approval of a conjugate vaccine that helps protect against 20 serotypes responsible for the majority of invasive pneumococcal disease and pneumonia, 1,2,3,4,5,6,7 including seven responsible for 40% of pneumococcal disease cases and deaths in the U.S. NEW YORK–(BUSINESS WIRE)– Pfizer Inc. Jansen, Ph.D.,

The Pharma Data
AUGUST 9, 2021
Research by Lancaster University scientists to create a COVID-19 vaccine which can be administered through the nose has taken a significant step forward. The pre-clinical animal trials of the intranasal vaccine showed a reduction in both the impact of the disease itself and transmission of the virus.

The Premier Consulting Blog
JUNE 18, 2023
One exciting application of these technologies is the use of in silico trials in the development of novel therapies for rare diseases. However, with rare diseases there may be no available treatments that could serve as SoC or active control in a clinical trial and assigning patients to placebo may be unethical.

The Pharma Data
MARCH 3, 2022
The FDA decision is informed by the results of the Phase 2b proof-of-concept study of RSVpreF (NCT04032093), a global, double-blinded, placebo-controlled study that assessed the safety and immunogenicity of RSVpreF in healthy pregnant women ages 18 through 49 years old, who were vaccinated between 28- and 36-weeks gestation, and their infants.

The Pharma Data
JUNE 26, 2021
BioNTech SE (NASDAQ: BNTX, “BioNTech” or “the Company”), announced that the first patient has been treated in its BNT111 Phase 2 cancer vaccine trial (2020-002195-12; NCT04526899). The trial is enrolling a total of 120 patients and will evaluate the effects of the combination as well as single agents alone.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content