How to advance AAV-based gene therapies
Drug Discovery World
OCTOBER 18, 2023
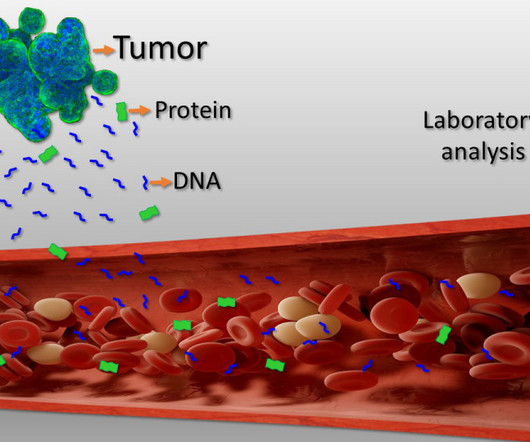
We’re beginning to see their potential come to fruition, with the FDA having approved three treatments as of January 2023 — for retinal dystrophy 1 spinal muscular atrophy, 2 and haemophilia B. Adeno-associated virus (AAV) vector-based gene therapies hold exceptional promise across a range of disease areas.








































Let's personalize your content