Three Principles for Writing Effective Analytics Documentation
Perficient: Drug Development
APRIL 11, 2024
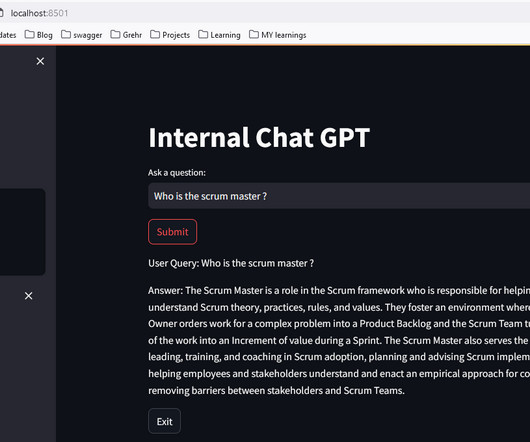
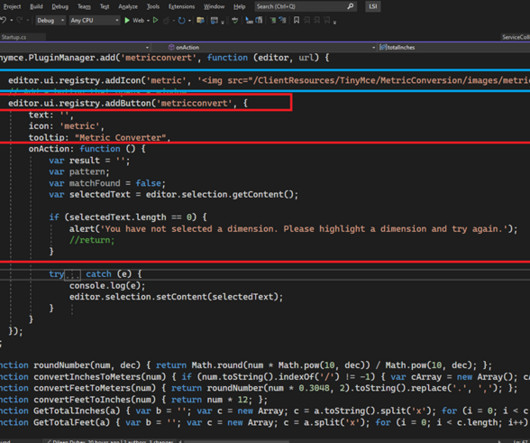
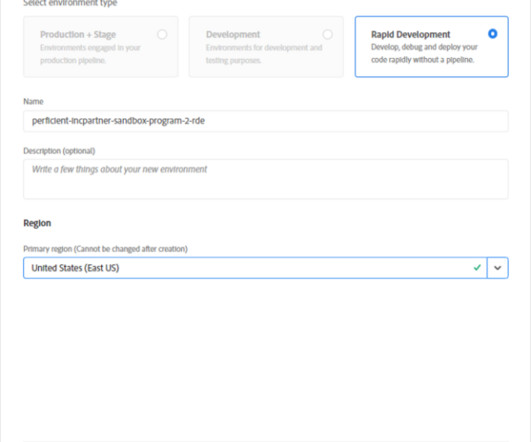
Documentation is a crucial part of your analytics implementation. From your Solution Design Reference document to your internal wiki for all resources, making sure that you can write and edit your documentation is key to ensuring a cohesive and thorough understanding of your implementation.














































Let's personalize your content