Using Rapid Development Environment in AEM as a Cloud Service
Perficient: Drug Development
NOVEMBER 10, 2023
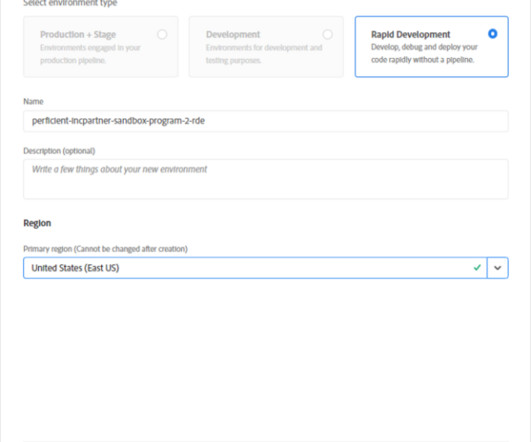
Depending on the size of the team and anticipated activity within a program, additional RDEs can also be provisioned, however, this may come at an additional licensing cost from Adobe. In this simple example, a content package is deployed with the aio aem:rde :install command.




























Let's personalize your content