Takeda secures FDA approval for colon cancer drug
BioPharma Drive: Drug Pricing
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.

ASPET
OCTOBER 12, 2023
To maintain cadence with looming threats in a prolonged field care environment, the broader medical countermeasure (MCM) enterprise must adopt new strategies for CBRN-addressing drug development. Repurposing is one such method.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The Pharma Data
JANUARY 17, 2021
plans to create a global R&D achievement based on innovations of inflammation–fibrosis treatment, Triple-acting new drug for NASH (non-alcoholic steatohepatitis) treatment as well as various other innovations in metabolic disease, oncology and rare disease fields. are expected to be approved by the U.S. . U.S.

The Pharma Data
NOVEMBER 8, 2020
FDA Approves Sesquient (fosphenytoin sodium) for the Treatment of Status Epilepticus in Adult and Pediatric Patients. Food and Drug Administration (FDA) has approved Sesquient (fosphenytoin sodium for injection) for the treatment of status epilepticus in adult and pediatric patients.

The Pharma Data
MAY 6, 2022
NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced they have submitted a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) to expand the approval of COMIRNATY® (COVID-19 Vaccine, mRNA) to include individuals ages 12 through 15 years. Pfizer Inc.
The Pharma Data
AUGUST 15, 2021
Food and Drug Administration (FDA) has approved TICOVAC (tick-borne encephalitis (TBE) vaccine) for active immunization to prevent TBE in individuals 1 year of age and older. 1 TICOVAC is the only FDA-approved vaccine to help protect U.S. 1 TICOVAC is the only FDA-approved vaccine to help protect U.S.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. Food and Drug Administration (“FDA”) has approved Danyelza (naxitamab-gqgk) 40mg/10ml. The product has received Priority Review, Orphan Drug, Breakthrough Therapy, and Rare Pediatric Disease designations from the FDA.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

The Pharma Data
DECEMBER 13, 2020
Food and Drug Administration (FDA) on Friday, December 11, 2020. During the meeting, the FDA provided encouraging feedback regarding the Phase 3 study of omidubicel pertaining to the pre-specified primary and secondary endpoints. Food and Drug Administration or any other health authority. About GDA-201.

The Pharma Data
JANUARY 15, 2021
Food and Drug Administration (FDA) approval of Darzalex Faspro ® (daratumumab and hyaluronidase-fihj), a subcutaneous formulation of daratumumab, in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd) for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis.[1] Haematologica.

The Pharma Data
DECEMBER 16, 2020
Recent correspondence between the Company and the FDA resulted in modifications to the previously disclosed trial design, including designating overall survival (OS) as the primary endpoint of the study. CNS Pharmaceuticals is developing novel treatments for primary and metastatic cancers of the brain and central nervous system.

The Pharma Data
AUGUST 21, 2020
Janssen acquired the worldwide license to develop, manufacture and commercialise the drug through an agreement with Genmab in 2012. This treatment has been approved following the randomised, open-label Phase 3 CANDOR trial that included 436 multiple myeloma patients who had relapsed.

The Pharma Data
JANUARY 24, 2021
Patents Related to P-selectin Expand Patent Life for Quercis’ Investigational Drug to Prevent and Treat Venous Thromboembolism in Cancer Patients and for Prevention and Treatment of COVID-19. Quercis’ lead drug candidate acts as an antithrombotic with significantly lower risk of adverse events than existing therapies.

The Pharma Data
MAY 13, 2021
Indian states turn to anti-parasitic drug to fight COVID-19 against WHO advice ( Reuters ). Japan vaccine chief blames drug approval system for slow inoculation drive ( Reuters ). Vaccine waiver talks can make drug firms the heroes, US trade chief says ( Reuters ) ( Law360 ).

FDA Law Blog: Biosimilars
JANUARY 18, 2024
Houck — In August 2023 the Food and Drug Administration (“FDA”) and Health and Human Services (“HHS”) recommended that the Drug Enforcement Administration (“DEA”) reschedule marijuana from schedule I under the federal Controlled Substances Act (“CSA”) to schedule III. The drug’s history and current pattern of abuse.

The Pharma Data
JUNE 18, 2021
Food and Drug Administration (FDA) has approved PREVNAR 20 (Pneumococcal 20-valent Conjugate Vaccine) for the prevention of invasive disease and pneumonia caused by the 20 Streptococcus pneumoniae (pneumococcus) serotypes in the vaccine in adults ages 18 years and older. Following today’s FDA approval, the U.S.

The Pharma Data
DECEMBER 16, 2020
NASDAQ: AUPH / TSX:AUP) (“Aurinia” or the “Company”) today announced it has entered into a collaboration and license agreement with Otsuka Pharmaceutical Co., Food and Drug Administration (FDA) with an assigned Prescription Drug User Fee Act (PDUFA) target action date of January 22, 2021.

The Pharma Data
OCTOBER 9, 2021
The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the submission of a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) seeking expanded approval of STELARA® (ustekinumab) to treat pediatric patients ages 5 years and older with juvenile psoriatic arthritis (jPsA).

The Pharma Data
OCTOBER 14, 2020
Food and Drug Administration (FDA) has approved WAKIX® (pitolisant) for the treatment of cataplexy in adult patients with narcolepsy. Drug Enforcement Administration. Drug Enforcement Administration. WAKIX is administered orally, once daily in the morning upon wakening.
New Drug Approvals
SEPTEMBER 8, 2024
1] [2] [3] The compound was licensed from Janssen Pharmaceutica NV. [4] 4] Seladelpar was approved for medical use in the United States in August 2024. [1] 1] [2] [3] The compound was licensed from Janssen Pharmaceutica NV. [4] 4] Seladelpar was approved for medical use in the United States in August 2024. [1]

The Pharma Data
OCTOBER 26, 2020
FDA Actions. FDA Approval: Last week the FDA approved Veklury (remdesivir) for the treatment of COVID-19 requiring hospitalization in adults and pediatric patients (12 years of age and older). Food and Drug Administration greenlit the restart of AstraZeneca’s Phase III COVID-19 vaccine trial, the U.K.-based

New Drug Approvals
DECEMBER 19, 2022
FDA 12/1/2022, To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, Rezlidhia Olutasidenib , sold under the brand name Rezlidhia , is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia. [1] 1] It is taken by mouth. [1] Hz, 1 H), 4.62−4.75

The Pharma Data
NOVEMBER 30, 2020
1, 2020 /PRNewswire/ — Sosei Group Corporation (“the Company”) (TSE: 4565) announces it has entered into a global collaboration and license agreement with Biohaven Pharmaceutical Holding Company Ltd. (“Biohaven”, NYSE: BHVN). . TOKYO and CAMBRIDGE, England , Dec. Vlad Coric , M.D.,
The Pharma Data
AUGUST 17, 2021
approval of pump use for Lilly’s novel insulin is latest development designed to help people with diabetes manage blood sugar levels. Lyumjev ® and Humalog ® are registered trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.

The Pharma Data
JANUARY 18, 2021
In total, there were 31 mostly virtual expert panel meetings in 2020, but more than a dozen of those did not involve votes on New Drug Applications (NDAs), Biologics License Applications (BLAs) or new indications, but instead focused on devices, tobacco or other topics. Jason Scott. Source link.

The Pharma Data
MAY 4, 2021
Lilly is offering donations of baricitinib to the Indian government through Direct Relief while simultaneously working with local Indian pharmaceutical companies to execute royalty-free voluntary licensing agreements to accelerate the manufacturing and distribution of the medicine in India during the pandemic.

The Pharma Data
DECEMBER 20, 2020
Food and Drug Administration (FDA) is plenty busy with COVID-19 vaccine Emergency Use Authorizations (EUAs) this month, but they’re also wrapping up the year with a few PDUFA dates for other therapies. The drug is a once-daily, beta-3 adrenergic agonist. Here’s a look. Urovant Sciences’ Vibegron for Overactive Bladder.

The Pharma Data
APRIL 14, 2021
Food and Drug Administration (FDA) has approved the company’s supplemental Biologics License Application for Xolair® (omalizumab) prefilled syringe for self-injection across all approved U.S. with Xolair since its initial approval in 2003. today announced that the U.S. indications.
NIH Director's Blog: Drug Development
JUNE 14, 2016
Several years ago, the Food and Drug Administration (FDA) recommended that drug developers take special care to show that potential drugs to treat diabetes don’t adversely affect the cardiovascular system [1]. In fact, the evidence suggests that such drugs might even offer some protection against heart disease.

New Drug Approvals
SEPTEMBER 6, 2024
2 , 3 Lazertinib was first approved in South Korea on January 18, 2021, for the treatment of EGFR T790M mutation-positive non-small cell lung cancer (NSCLC) with EGFR mutations. 1 It was approved by the FDA on August 19, 2024. Food and Drug Administration (FDA). 19 August 2024. Retrieved 21 August 2024.
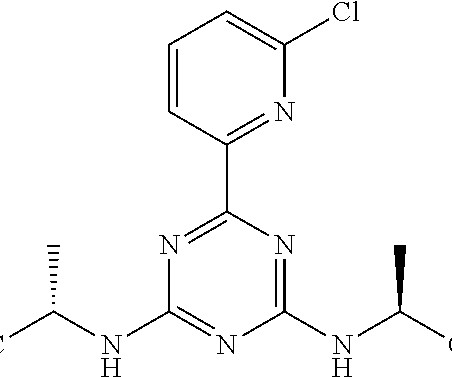
New Drug Approvals
SEPTEMBER 10, 2024
2] Vorasidenib was approved for medical use in the United States in August 2024. [2] 2] [3] It is the first approval by the US Food and Drug Administration (FDA) of a systemic therapy for people with grade 2 astrocytoma or oligodendroglioma with a susceptible isocitrate dehydrogenase-1 or isocitrate dehydrogenase-2 mutation. [2]

FDA Law Blog: Biosimilars
JULY 5, 2023
Palmer — Last week FDA published a long-awaited Draft Guidance for outsourcing facilities addressing the Prohibition on Wholesaling Under Section 503B of the Federal Food, Drug, and Cosmetic Act (Draft Guidance). See 21 U.S.C. 353b(a)(8). Section II at 2.

FDA Law Blog: Biosimilars
AUGUST 8, 2024
With apologies to Curtis Mayfield) By the close of the public comment period for the Drug Enforcement Administration’s (“DEA’s”) proposal to reschedule marijuana two weeks ago, the agency had received over 43,500 comments.

FDA Law Blog: Biosimilars
FEBRUARY 26, 2024
Relevant here, FDA interpreted in Guidance that a proposed injectable biosimilar must “demonstrate that its product has the same strength as the reference product by demonstrating that both products have the same total content of drug substance (in mass or units of activity) and the same concentration of drug substance.”

The Pharma Data
FEBRUARY 25, 2022
Food and Drug Administration (FDA) has accepted for review the Prior Approval Supplement (PAS) to the Biologics License Application (BLA) for ABRILADA™ (adalimumab-afzb) as an interchangeable biosimilar to Humira® (adalimumab). The Biosimilar User Fee Act (BsUFA) goal date for an FDA decision is in Q4 2022.
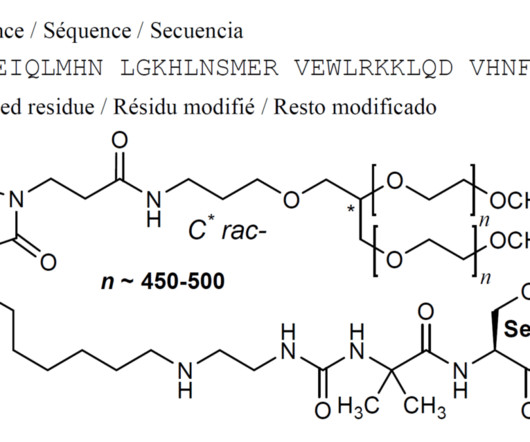
New Drug Approvals
SEPTEMBER 8, 2024
1] Palopegteriparatide was approved for medical use in the European Union in November 2023, [2] and in the United States in August 2024. [1] 5] Study drug and conventional therapy were subsequently adjusted according to the albumin-corrected serum calcium levels. [5] Food and Drug Administration (FDA) (Press release).
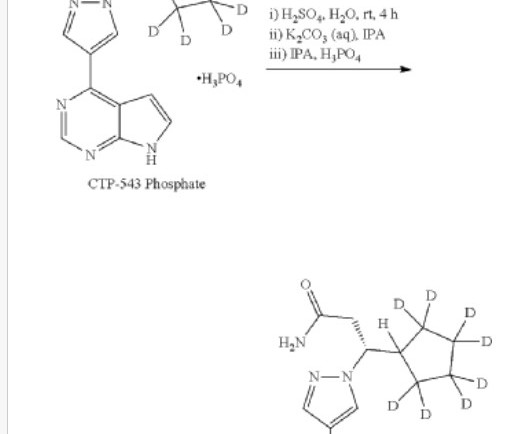
New Drug Approvals
SEPTEMBER 10, 2024
Deuruxolitinib C 17 H 18 N 6 , 314.422 Fda approved Leqselvi , 7/25/2024, To treat severe alopecia areata C-21543, CTP 543, CTP-543, CTP543 (3r)-3-(2,2,3,3,4,4,5,5-d8)cyclopentyl-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)propanenitrile 1h-pyrazole-1-propanenitrile,beta.-(cyclopentyl-2,2,3,3,4,4,5,5-d8)-4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-,
Codon
JULY 14, 2024
Despite the initial promise of PFCs and FDA approval of a product called Fluosol-DA in 1989, PFCs have many drawbacks. Hemopure, the only FDA-approved HBOC product, is a bovine hemoglobin that is chemically crosslinked (that is, several hemoglobin molecules are bound to each other) to improve its stability.

The Pharma Data
AUGUST 17, 2021
Second FDA approved indication for dostarlimab in 2021 GARNET study demonstrated objective response rate of 41.6% This indication received accelerated approval based on tumour response rate and durability of response. This approval was based on data from cohort A1, which included 71 patients with dMMR endometrial cancer. [vii]

The Pharma Data
APRIL 4, 2022
If approved, Actemra/RoActemra would be the first U.S. FDA approval is expected in the second half of this year. Food and Drug Administration (FDA)-approved for this use and there is limited information known about the safety or effectiveness of using Actemra/RoActemra to treat people in the hospital with COVID-19.

New Drug Approvals
DECEMBER 24, 2023
S2CID 250989659. ^ “Eplontersen: FDA-Approved Drugs” U.S. Food and Drug Administration (FDA). Retrieved 21 December 2023. ^ “Wainua (eplontersen) granted regulatory approval in the U.S. . | twitter +919321316780 call whatsaapp EMAIL. 88 (12): 5389–5398. doi : 10.1111/bcp.15468.

The Pharma Data
NOVEMBER 22, 2020
Food and Drug Administration (FDA) has a busy end of November planned, with numerous PDUFA dates to address. The FDA approved it under the brand name Gavreto on September 4. The drug is a once-daily oral precision therapy designed for highly potent and selective targeting of oncogenic RET alterations.

The Pharma Data
NOVEMBER 9, 2021
TERMS OF THE ARRANGEMENT Biohaven and Pfizer are entering into a collaboration and license agreement and affiliated sublicense agreement pursuant to which Pfizer will acquire rights to manipulate rimegepant and zavegepant outside of theU.S. Rimegepant was approved by theU.S. and coequals outside theU.S.,

New Drug Approvals
SEPTEMBER 20, 2023
1] Motixafortide was approved for medical use in the United States in September 2023. [2] 4 Similar in mechanism to the previously approved plerixafor , motixafortide is an inhibitor of C-X-C Motif Chemokine Receptor 4 (CXCR4), a protein that helps to anchor stem cells to bone marrow matrix. 1] It is given by subcutaneous injection. [1]
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content