Pfizer wins FDA approval for its $7B colitis drug
BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.

Drugs.com
JANUARY 31, 2025
Food and Drug Administration (FDA) has approved Journavx, a new pain reliever without the risks of addiction or overdose linked to drugs like Vicodin and OxyContin.The new pill, developed by Vertex Pharmaceuticals. FRIDAY, Jan. 31, 2025 -- The U.S.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Patent Watch
JULY 24, 2024
In the high-stakes world of pharmaceuticals, generic drugs have become the unsung heroes of healthcare accessibility. These cost-effective alternatives to […] Source

SCIENMAG: Medicine & Health
OCTOBER 31, 2023
— A new drug developed by professors from the School of Pharmacy and Pharmaceutical Sciences at Binghamton University has received Food and Drug Administration (FDA) approval for the treatment of patients with Duchenne muscular dystrophy (DMD), a common genetic disease that mostly affects young boys.

The Pharma Data
JANUARY 17, 2021
18 , 2021 /PRNewswire/ — Hanmi Pharmaceutical Co., plans to create a global R&D achievement based on innovations of inflammation–fibrosis treatment, Triple-acting new drug for NASH (non-alcoholic steatohepatitis) treatment as well as various other innovations in metabolic disease, oncology and rare disease fields.

The Pharma Data
NOVEMBER 8, 2020
FDA Approves Sesquient (fosphenytoin sodium) for the Treatment of Status Epilepticus in Adult and Pediatric Patients. PAOLI, Pa., — (BUSINESS WIRE) — November 9, 2020 – Sedor Pharmaceuticals, LLC (Sedor) today announced that the U.S. About Sedor Pharmaceuticals, LLC.
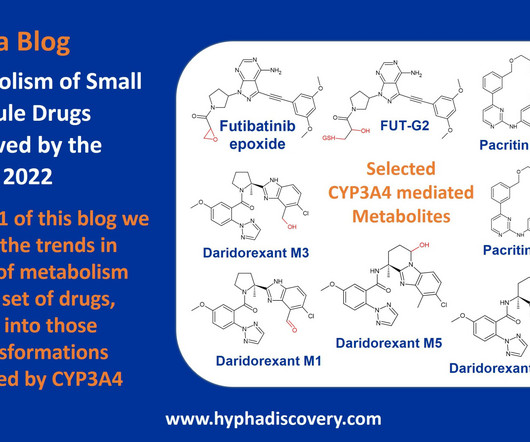
Metabolite Tales Blog
JANUARY 26, 2023
Metabolism of 2022 FDA approved small molecule drugs – Part 1 Does CYP3A4 still rule? By Julia Shanu-Wilson It won’t come as much surprise to learn that of the 17 small molecules* approved by the FDA in 2022, CYP3A4 was the major player in drug metabolism.

The Pharma Data
JANUARY 17, 2021
Food and Drug Administration (FDA) approved Janssen Pharmaceuticals ’ (a Johnson and Johnson company) Darzalex Faspro for adults with newly diagnosed light chain amyloidosis. It was approved in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd). Michael Vi/Shutterstock.

The Pharma Data
DECEMBER 16, 2020
17, 2020 /PRNewswire/ — CNS Pharmaceuticals, Inc. Recent correspondence between the Company and the FDA resulted in modifications to the previously disclosed trial design, including designating overall survival (OS) as the primary endpoint of the study. About CNS Pharmaceuticals, Inc.

The Pharma Data
AUGUST 27, 2020
Food and Drug Administration (FDA) has approved FoundationOne®Liquid CDx, Foundation Medicine’s comprehensive pan-tumour liquid biopsy test for patients with solid tumours. The test is FDA-approved to report short variants in 311 genes including rearrangements and copy number losses in BRCA1 and BRCA2 genes.

FDA Law Blog: Biosimilars
OCTOBER 1, 2024
Karst — If you monitor Regulations.gov dockets and litigation dockets on PACER like we do, then you know that one company name—more than any other over the past several years—pops up: Vanda Pharmaceuticals, Inc. 2) because the FDA employees who approved the application were not “Officers of the United States.” II, § 2, cl.

Drug Target Review
JANUARY 8, 2024
We are at the forefront of drug development in an area of research called cellular rejuvenation, which is an approach that has the potential to address many diseases of ageing by restoring aged and injured cells to a more youthful and resilient state. What evidence is there that we can reverse aging with drugs?

The Pharma Data
NOVEMBER 2, 2020
FDA Approves Bronchitol (mannitol) Inhalation Powder to Improve Pulmonary Function in Adult Patients with Cystic Fibrosis. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder.
The Pharma Data
JUNE 26, 2021
Food and Drug Administration approved Pradaxa (dabigatran etexilate) oral pellets to treat children 3 months to less than 12 years old with venous thromboembolism (a condition where blood clots form in the veins) directly after they have been treated with a blood thinner given by injection for at least five days.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Imcivree (setmelanotide) for Chronic Weight Management in Patients with Obesity Due to POMC, PCSK1 or LEPR Deficiency. 27, 2020 (GLOBE NEWSWIRE) — Rhythm Pharmaceuticals, Inc. With this approval, Imcivree becomes the first-ever FDA approved therapy for these rare genetic diseases of obesity.

Codon
NOVEMBER 3, 2024
As an undergraduate biology student, I spent some time in a TB lab working on antibiotic resistance — a growing concern for drug developers. Antibiotics From the Ground Up Researchers unearthed the first TB drug from the ground. We need better vaccines — and medicines — to quash TB.

Pharmaceutical Development Group
AUGUST 9, 2021
Formulation chemistry is the systematic and step-by-step approach to pharmaceutical development. Nowadays, as the usage of medicines is increasing, pharmaceutical companies are more eager to bring manufacturing a new look in quality and performance. There are different tests to assess the quality of a pharmaceutical product.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. Food and Drug Administration (“FDA”) has approved Danyelza (naxitamab-gqgk) 40mg/10ml. The product has received Priority Review, Orphan Drug, Breakthrough Therapy, and Rare Pediatric Disease designations from the FDA.

The Pharma Data
NOVEMBER 24, 2020
FDA Approves Oxlumo (lumasiran) for the Treatment of Primary Hyperoxaluria Type 1. 24, 2020– Alnylam Pharmaceuticals, Inc. Oxlumo demonstrated an encouraging safety and tolerability profile, with injection site reactions (ISRs) as the most common drug-related adverse reaction. CAMBRIDGE, Mass.–(BUSINESS

The Pharma Data
SEPTEMBER 6, 2020
Basel, 7 September 2020 – Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that the United States (US) Food and Drug Administration (FDA) has approved Gavreto (pralsetinib) for the treatment of adults with metastatic rearranged during transfection (RET) fusion-positive non-small cell lung cancer (NSCLC) as detected by an FDA approved test.

Advarra
JULY 27, 2022
As a pharmaceutical product makes its way through the lifecycle, there are often Food and Drug Administration (FDA) guidelines organizations should pay particular attention to. If your go-to-market strategy requires FDA approval, it may also require a prior approval supplement. Go-to-market Strategy.
Drug Target Review
JUNE 5, 2023
Can you tell us more about ZW191 and its potential as an FRα-targeting antibody-drug conjugate? ZW191 is comprised of a novel fully humanised IgG1 antibody covalently conjugated to a novel topoisomerase 1 inhibitor ZD06519, a camptothecin derivative, via endogenous interchain cysteines with a drug-to-antibody ratio (DAR) of eight.

The Pharma Data
JANUARY 15, 2021
January 15, 2021 (HORSHAM, Pa.) – The Janssen Pharmaceutical Companies of Johnson & Johnson announced today the U.S. 2],[3] This indication is approved under accelerated approval and is based on the hematologic complete response rate (hemCR) measure. Approximately 4,500 people in the U.S.

The Pharma Data
MAY 27, 2022
Novartis today announced the US Food and Drug Administration (FDA) has granted accelerated approval for Kymriah ® (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.

The Pharma Data
APRIL 19, 2022
Food and Drug Administration (FDA) has approved commercial production at the company’s new CAR T-cell therapy manufacturing facility in Frederick, Maryland. The site will produce Kite’s FDA approved CAR T-cell therapy used to treat blood cancer. Kite, a Gilead Company (Nasdaq: GILD), today announced the U.S.

Eye on FDA
AUGUST 4, 2021
As more drugs are being approved, is FDA getting less advice than in the past? FDA maintains a vast network of outside advisors to provide input and counsel to the agency related to decisions on policy as well as product approvals. Fewer meetings – more approvals.

The Pharma Data
MARCH 15, 2022
Agency Supports Development of Complex Generic Drug-Device Combination Product to Improve Competition and Access to More Affordable Medicines. This complex generic drug-device combination product, which is a metered-dose inhaler, should not be used to treat acute asthma attacks. Today, the U.S.

The Pharma Data
FEBRUARY 25, 2022
Jardiance is the first and only heart failure therapy to demonstrate a statistically significant risk reduction in cardiovascular death or hospitalization for heart failure, regardless of ejection fraction FDA approval marks a significant breakthrough for the approximately 3 million adults in the U.S. and Europe. and Europe.
The Pharma Data
NOVEMBER 2, 2020
GW Pharmaceuticals hopes to bring its cannabis-based treatment for multiple sclerosis spasticity to the United States. Sativex is approved for use in parts of Europe for this indication. Justin Gover, chief executive officer of GW Pharmaceuticals, proclaimed his excitement about launching the Phase III study in the United States.

The Pharma Data
NOVEMBER 23, 2021
Approval is for Cytomegalovirus, a Type of Herpes Virus. director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern. Today, the U.S. It’s a type of herpes contagion.

The Pharma Data
JANUARY 6, 2021
The research collaboration is aimed at identifying novel lead compounds and repurposing existing drugs for rheumatoid arthritis and nonalcoholic steatohepatitis, leveraging Standigm’s AI-powered drug discovery platforms : Standigm BEST , Standigm Insight , and Standigm ASK. Standigm is an AI-driven drug discovery company.
The Pharma Data
JULY 29, 2021
Food and Drug Administration approved the first interchangeable biosimilar insulin product, indicated to improve glycemic control in adults and pediatric patients with Type 1 diabetes mellitus and in adults with Type 2 diabetes mellitus. director of the Office of New Drugs in the FDA’s Center for Drug Evaluation and Research.

FDA Law Blog: Biosimilars
JUNE 27, 2023
Valentine Named Top Lawyer Under 40; Only Food and Drug Lawyer Selected Hyman, Phelps & McNamara, P.C. (HP&M) James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list. HP&M’s James E. Valentine , as a 2023 Rising Star.

The Pharma Data
MAY 18, 2021
Food and Drug Administration (FDA) approval of the VENTANA MMR RxDx Panel for advanced or recurrent endometrial cancer patients. FDA approval of the VENTANA MMR RxDx Panel provides clinicians with access to a fully automated, easy-to-use MMR test to identify patients who are eligible for therapy with JEMPERLI.
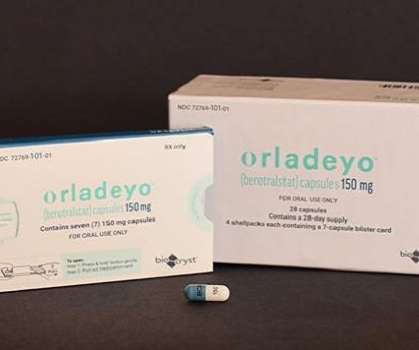
The Pharma Data
DECEMBER 3, 2020
BioCryst Pharmaceuticals, Inc. Food and Drug Administration (FDA) has approved ORLADEYO (berotralstat) for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and young patients 12 years and older. announced on Thursday that the U.S.

The Pharma Data
AUGUST 31, 2020
Food and Drug Administration (FDA) approval for the cobas® HIV-1/HIV-2 Qualitative Test for use on the fully automated cobas® 6800/8800 Systems in the U.S. About Roche Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. Learn more now: www.cobas68008800.com.
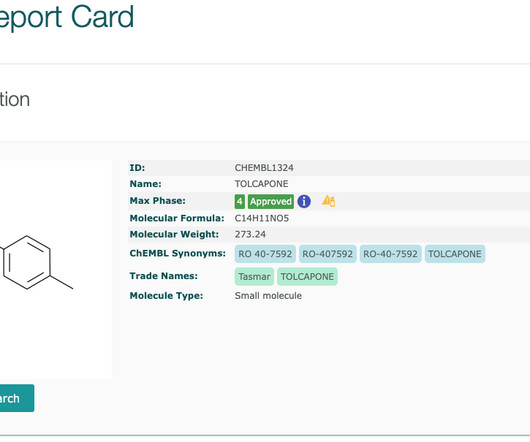
The ChEMBL-og
FEBRUARY 22, 2021
Updated drug safety information is available (as of ChEMBL 28 ) for drugs with boxed warnings and for withdrawn drugs. Boxed warnings (also know as black box warnings) are provided on medicinal product labels for FDA approved drugs if the medicinal product can cause severe or life-threatening side effects.

The Pharma Data
MAY 6, 2022
Food and Drug Administration (FDA) to expand the approval of COMIRNATY® (COVID-19 Vaccine, mRNA) to include individuals ages 12 through 15 years. Food and Drug Administration (FDA) authorized on May 10 , 2021. Pfizer Inc. This is the only COVID-19 vaccine authorized for this age group in the U.S.
The Pharma Data
SEPTEMBER 1, 2021
Approval is backed by nearly two decades of proven efficacy and safety of Janssen’s long-acting injectable portfolio of schizophrenia medicines. The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the U.S.

The Connected Lab
APRIL 8, 2020
Prescription drugs have the ability to transform a patient’s life and provides them the opportunity to rid a devastating illness, making the development and approval of these medications urgent and necessary. However, over the last 10 years the path leading to drug approval has become more complicated and expensive.
KIF1A
JULY 1, 2023
Read the Pre-print Rare Roundup A seemingly small semantic issue is a major roadblock to develop treatments for rare diseases We’ve spoken often about the goal of repurposing existing drugs to better treat KAND – this is a reality for many families who utilize off-label prescriptions.

The Pharma Data
MAY 31, 2022
Food and Drug Administration (FDA) has approved a label extension for Evrysdi® (risdiplam) to include babies under two months old with spinal muscular atrophy (SMA). Evrysdi was granted PRIME designation by the European Medicines Agency (EMA) in 2018 and Orphan Drug Designation by the U.S

Drug Target Review
SEPTEMBER 19, 2023
The US FDA Modernisation Act 2.0., puts an end to the previous mandate that all drugs need to be tested on animals prior to human clinical trials. 3-5 Boston biotech, Emulate, established liver-on-chip device is designed to accurately measure liver toxicity and predict drug induced liver injury (DILI).

The Pharma Data
JULY 8, 2021
Food and Drug Administration (FDA) has approved an updated label for ADUHELM (aducanumab-avwa) injection 100 mg/mL solution. is a leading global pharmaceutical company headquartered in Japan. ADUHELM should be initiated in patients with mild cognitive impairment due to Alzheimer’s disease or mild Alzheimer’s dementia.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content