Ventyx’s TYK2 drug suffers second setback
BioPharma Drive: Drug Pricing
JULY 29, 2024
The company claimed “higher than anticipated” scores from trial volunteers on placebo led to the disappointing data, prompting it to halt internal trials.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
JULY 29, 2024
The company claimed “higher than anticipated” scores from trial volunteers on placebo led to the disappointing data, prompting it to halt internal trials.

Drug Patent Watch
DECEMBER 9, 2024
This intersection is where pharmacognosy meets drug patents, creating a unique landscape that shapes the future of medicine. But what exactly is pharmacognosy, and how does it relate to the complex world of drug patents? This approach has led to the discovery of numerous potential drug candidates. What is a Drug Patent?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
New Drug Approvals
APRIL 11, 2025
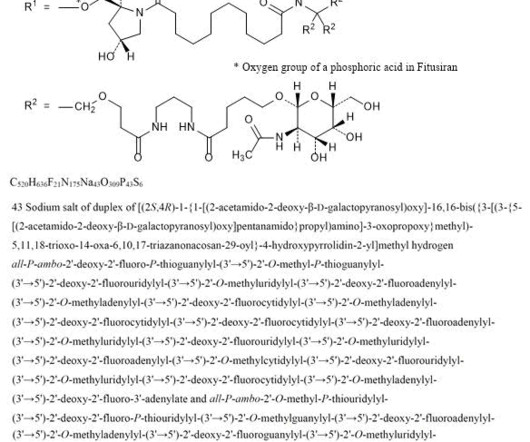
1] [2] Adverse effects The US Food and Drug Administration prescription label for fitusiran contains a boxed warning for thrombotic events (blood clotting) and gallbladder disease (with some recipients requiring gallbladder removal). [2] 2] [3] Names Fitusiran is the international nonproprietary name. [4] Food and Drug Administration.

Drug Target Review
APRIL 7, 2025
A surrogate endpoint is a marker used in clinical trials as a substitute for a direct clinical outcome. Diagnostic biomarkers typically confirm or establish a diagnosis and are often used in selecting patient populations for clinical trials.
PPD
OCTOBER 31, 2024
As the pharmaceutical industry continues to evolve, drug developers encounter new challenges and opportunities in their pursuit of innovation. From adapting to complex new trial designs to embracing cutting-edge technologies, staying ahead requires a deep understanding of the current landscape.

Drug Patent Watch
DECEMBER 30, 2024
The regulatory environment in Japan for generic drug development is complex and has undergone significant changes in recent years. Regulatory Authority: Pharmaceuticals and Medical Devices Agency (PMDA) The PMDA is the primary regulatory authority responsible for overseeing the drug approval process in Japan.

PPD
DECEMBER 16, 2024
Our annual look at the state of the drug development industry highlights a dual set of challenges complicating progress. Pressure and requirements to engage diverse patient populations in trials have become more challenging and expensive, requiring tailored strategies that can stretch both resources and budgets.

PPD
OCTOBER 21, 2024
Meeting the never-ending challenges of drug development in this active environment — including pressure to identify drug prospects earlier and hire more conservatively — frequently leads biotech companies to outsource some portion of clinical development functions. With FSO, all tasks for a clinical trial are outsourced.
Drug Target Review
OCTOBER 30, 2024
Now take a step further: envision testing drugs in these organoids to identify the ones that can treat disease safely and effectively without needing to run expensive clinical trials first. 12 Testing drugs in vitro in organoid cultures A clear validation of the utility of organoids is in drug discovery and development.
PPD
JUNE 7, 2023
Food and Drug Administration (FDA) for approval , much work remains for drug developers aiming to advance treatments for pediatric populations. Reflecting Patient Diversity in NASH Trials Data from NASH clinical trials within adult populations may support clinical research into treatment for pediatric populations.

PPD
AUGUST 5, 2024
Historically, the available drugs and U.S. Food and Drug Administration (FDA)-approved therapies for treating PAH were primarily vasodilators, designed to overcome the imbalance between vasoactive and vasodilator mediators and to restore endothelial cell function.

Drug Discovery Today
NOVEMBER 10, 2022
A team of surgeons and scientists from the UK, Sweden and Canada, funded by Rinri Therapeutics, has confirmed secure surgical access to the central core of the human cochlea The research, published in Scientific Reports, is critical to the first in-human trials of new cell, gene and drug therapies for the inner ear, and will assist with treatment for (..)

Conversations in Drug Development Trends
AUGUST 9, 2024
Authors: Matt Cooper, PhD, Executive Director, Therapeutic Strategy Lead, Oncology; Megan Morrison, Vice President, Asia Pacific Strategy Lead Adaptive trial designs have become essential in oncology, offering a flexible and efficient approach for conducting clinical trials.
Conversations in Drug Development Trends
APRIL 3, 2024
Are you aware of the challenges you must address for a successful radiopharmaceutical trial? Enhancing Patient Participation in Radiopharmaceutical Trials Patient recruitment is a critical yet challenging part of radiopharmaceutical trials.

Drug Target Review
FEBRUARY 10, 2025
In just two years, CTMC has advanced eight therapies into clinical trials, harnessing genetic engineering to enhance T-cell effectiveness in the fight against cancer. Unlike traditional drug manufacturing, where the supply chain is linear, autologous cell therapies involve a more complex, circular process.

Drug Target Review
DECEMBER 11, 2024
This shift in focus is especially critical in toxicology, where accurate target analysis plays a vital role in identifying toxic effects and ensuring patient safety, particularly as the field transitions from traditional drugs to the promising realm of biotherapeutics, especially for rare diseases.

Conversations in Drug Development Trends
JANUARY 24, 2024
Written By: Derek Ansel, MS, CCRA, Executive Director, Therapeutic Strategy Lead, Rare Disease Given that 80% of rare diseases have a genetic etiology, genetic implications should be addressed at the onset of a clinical program to support trial enrollment. One diagnostic example that I discussed in my presentation is autism.

National Institute on Drug Abuse: Nora's Blog
MARCH 6, 2025
Advancing reduction of drug use as an endpoint in addiction treatment trials astewart Thu, 03/06/2025 - 09:59 Nora's Blog March 18, 2025 Image Getty Images/ SolStock This blog was also published in the American Society of Addiction Medicine (ASAM) Weekly on March 18, 2025.&

Drug Channels
SEPTEMBER 28, 2021
Curl up with your favorite pumpkin-spiced blog and savor these stories harvested from the Drug Channels patch: Fresh insights about hospitals’ specialty drug profits SSR Update: Drug prices keep dropping My $0.02 d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc.

Antidote
JUNE 22, 2023
Creating clinical trial marketing materials is a delicate balance — it's important to craft outreach that connects with patients and care partners, but it’s also important to adhere to the guidelines set out by the Food and Drug Administration (FDA) to receive the approval of the Internal Review Board (IRB).

Conversations in Drug Development Trends
MAY 1, 2024
This process can be daunting, but understanding how to manage feedback effectively is crucial for developing and ultimately gaining approval for new therapies, especially in oncology clinical trials. An illustrative example of harmonization between agencies exists via the European Medicines Agency (EMA) and U.S.

PPD
FEBRUARY 10, 2025
Sponsors find that an FSP solution is often the best choice to help advance their drug development projects, whether they need to fill small gaps in services or support large-scale programs with dedicated teams across functions. Electronic medical records (EMRs), for example, exemplify the evolving role of technology in clinical trials.

Alta Sciences
MARCH 4, 2025
The CCALC is a grassroots organization that was founded by several pharmaceutical industry members seeking clarity around the conduct of abuse and dependence potential assessments for novel drugs in development. I have spent the better part of my career working to make drugs safer. corticosteroids, beta-blockers, antidepressants).
New Drug Approvals
APRIL 2, 2025
2] [6] Suzetrigine is the first medication to be approved by the US Food and Drug Administration (FDA) in this new class of pain management medicines. [2] 2] [6] Suzetrigine is the first medication to be approved by the US Food and Drug Administration (FDA) in this new class of pain management medicines. [2]
Drug Channels
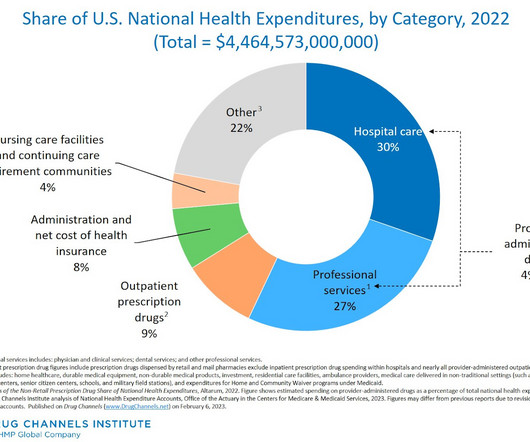
FEBRUARY 7, 2024
As you will see, retail and mail prescription drug spending remain a consistently small share of the $4.5 And contrary to what you might read, drug spending growth was *not* driven by purportedly “skyrocketing” drug prices. Tomorrow, we’ll be treated to a Senate show trial featuring pharmaceutical company executives.

Advarra
MARCH 6, 2025
For more than 35 years, Advarra has been committed to protecting the rights and welfare of clinical trial participants while helping to improve healthcare outcomes, advancing medical knowledge, and bringing innovative, life-extending treatments to market that benefit millions of patients worldwide.

PPD
SEPTEMBER 6, 2023
Approaches to outsourcing clinical trials have changed significantly in recent years. Mixing of service models — a strategy that drug developers are leveraging now more than ever — can bring life-changing therapies to market faster. Overseen by an insourced project manager. Contracts are milestone- or unit-based.

New Drug Approvals
APRIL 18, 2025
2] [3] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [4] kg, the preparation of Compound 8A has been previously described in International Publication Number WO2010/125414) were added to the reactor and heated to 85 C. 1] [2] [5] Names Crinecerfont is the international nonproprietary name. [6]

Vial
JUNE 12, 2024
PROs in clinical trials are important as they capture the patient’s perspective and ensure that the impact of an intervention is comprehensively evaluated. Food and Drug Administration (FDA) increasingly look to patients to understand how they describe their health status. What are PROs in clinical trials?
The Pharma Data
JULY 23, 2021
(Nasdaq: BIIB) today announced it will share multiple oral and poster presentations from its Alzheimer’s disease clinical development portfolio at the Alzheimer’s Association International Conference (AAIC), which will be held in Denver, Colorado and online July 26-30, 2021.

DrugBaron
JANUARY 31, 2024
Both the cost and the time required for each new drug approval have increased exponentially for decades (aptly tagged ‘Eroom’s Law’, being the inverse of price drops and speed gains in the semiconductor industry). So what about the theoretical basis for the claim that AI can “revolutionise” drug discovery and development?

Drug Target Review
SEPTEMBER 21, 2023
The findings supported the possibility put forward by Cotton et al (2016) concerning the chance of repurposing these drugs for adulticidal treatment. Need for adulticidal drugs More than 99 percent of countries worldwide has been affected by this disease. They provided a big stepping stone, needed for the development of drugs.

New Drug Approvals
APRIL 25, 2025
12] However, absorption, metabolism, and excretion data of taselisib , a molecule with a related chemical scaffold, suggest moderately slow absorption into the systemic circulation, metabolism to play a minor role in drug clearance, and biliary excretion to be the main route of excretion. [13] Food and Drug Administration (FDA).

Alta Sciences
AUGUST 21, 2024
Internships at Altasciences: Q&A With Our Summer Interns nbartlett Thu, 08/22/2024 - 04:44 Internships at Altasciences are more than just a stepping stone—they’re a gateway to real-world-experience and professional growth, giving the next generation opportunities to help shape progress in the drug development industry.
The Pharma Data
AUGUST 30, 2021
.–(BUSINESS WIRE)– Showcasing Teva’s Commitment to Helping Patients Have More Migraine-Free Days, 18 Abstracts will be presented, Including One Late-Breaker, on AJOVY ® (fremanezumab-vfrm) Injection at the International Headache Society and European Headache Federation Joint Congress 2021. Teva Pharmaceuticals USA, Inc.,

H1 Blog
JULY 19, 2023
Streamlining recruitment and reporting to support advanced oncology drug development In March of 2023, the U.S. In order to qualify for accelerated approval, drug manufacturers must demonstrate that the medication provides meaningful clinical benefit to patients with life-threatening illnesses during their clinical trials.
Advarra
JANUARY 13, 2023
This blog explores what it takes for an EAC/CEC to adequately support worldwide clinical trials. Having one set of adjudicators across the entire trial aids consistency of determinations and allows for a central decision-making framework. was it caused by the study drug or an underlying condition).

Advarra
JUNE 20, 2023
of new cancer drugs tested in Phase I were likely to receive Food and Drug Administration (FDA) approval. Typical clinical development timelines for anticancer drugs average an estimated 6.7 Innovation Organizations conducting oncology clinical trials face challenges distinct from the rest of the research community.

Advarra
OCTOBER 1, 2024
Managing clinical trial budgets efficiently is necessary for the success and sustainability of clinical research sites. Effective budget management not only ensures trials are financially viable but also maximizes return on investment (ROI). the impact and value of the data produced).

Drug Target Review
MARCH 17, 2025
Advancing drug candidates across key therapeutic areas Dr John Donello brings over 25 years of experience in pharmaceutical drug discovery, development and collaborations. An NMDA receptor modulator is a drug that targets the NMDA receptor in the brain, which is crucial for processes like synaptic plasticity, learning and memory.

Vial
MARCH 25, 2024
Introduction Worldwide Clinical Trials vs. Vial. Worldwide Clinical Trials is a mid-size, full-service global contract research organization (CRO) that works with biotech and pharma to advance new medications. With an international presence in nearly 60 countries, Worldwide is supported by over 3,400 team members.
The Pharma Data
SEPTEMBER 9, 2021
This data will be presented as a late-breaker ePoster during the International Headache Society (IHS) and European Headache Federation (EHF) Joint Congress taking place virtually on Sept. Adverse Reactions: The most common adverse reactions in clinical trials (?5% 8-12, 2021. Reactions have included anaphylaxis and angioedema.

Practical Cheminformatics
JANUARY 8, 2024
Picking up where we left off in Part I , this post covers several other ML in drug discovery topics that interested me in 2023. In November and December, several large pharmas held “AI Day” presentations featuring LLM applications for clinical trial data analysis. Most of the drug discovery examples were underwhelming.

The Pharma Data
SEPTEMBER 6, 2021
The favourable tolerability of eliapixant in this trial is consistent with earlier clinical findings. The selective mechanism of action of eliapixant appears to be translating into improved tolerability in clinical trials. Blood samples were collected to monitor safety and measure the blood level of the study drug.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content