What you should know about the FDA’s Biological License Application Process
Reprocell
SEPTEMBER 19, 2024
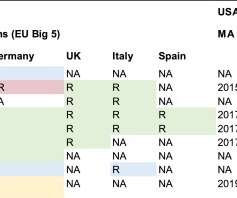
The biological license application (BLA) is one of the many requests for marketing approval received by the FDA. Unlike the New Drug Application (NDA) which is usually the go-to submission for chemically synthesized, low molecular weight drugs BLAs grant sponsors the ability to introduce Biologics into interstate commerce.













































Let's personalize your content