New drug triggers rapid cell death in cancer models
Broad Institute
OCTOBER 29, 2024
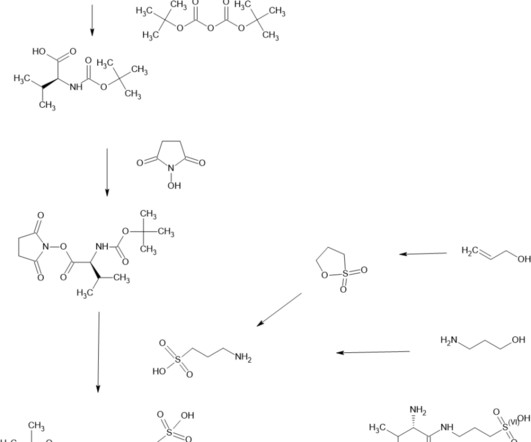
New drug triggers rapid cell death in cancer models By Karen Zusi-Tran October 29, 2024 Breadcrumb Home New drug triggers rapid cell death in cancer models BRD-810 inhibits the MCL1 protein and reactivates apoptosis in tumor cells, displaying therapeutic potential in animal models. The result was the compound named BRD-810.
















































Let's personalize your content