Concurrent Validation for Breakthrough and Orphan Drugs: Meeting the Needs for Accelerated Manufacturing
The Premier Consulting Blog
NOVEMBER 20, 2024
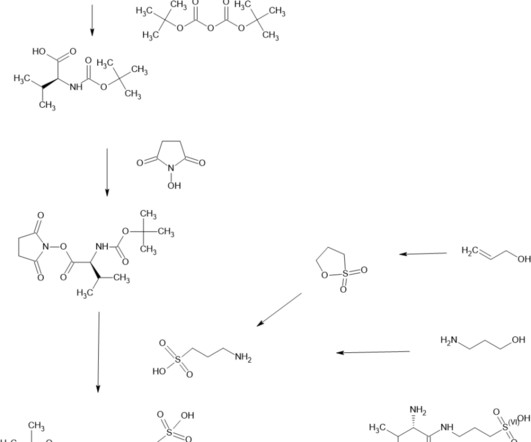
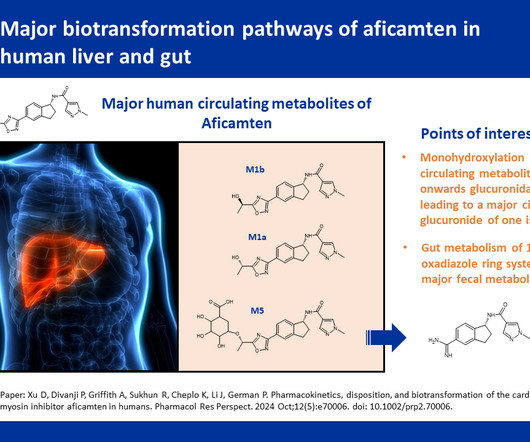
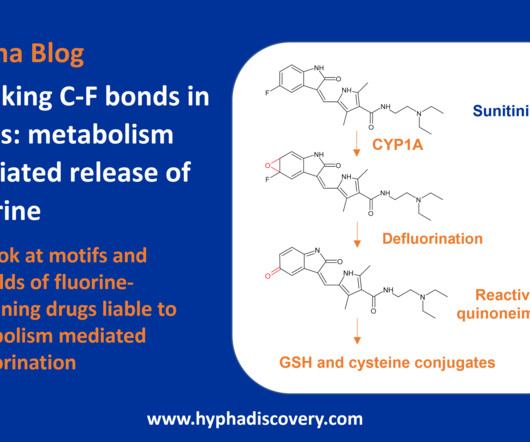
In the case of urgent or immediate public interest, process validation may be conducted concurrently with manufacturing the commercial small molecule or biologic product to expedite product availability for patients. It ensures that a production process consistently yields products of predetermined quality and safety.










































Let's personalize your content