Unlocking European market access for rare disease therapies
Fierce BioTech
SEPTEMBER 24, 2024
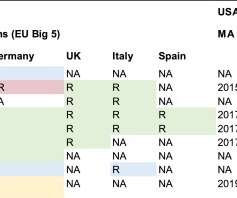
Unlocking European market access for rare disease therapies The European orphan drug market presents a significant opportunity for biotech firms. tharilall Tue, 09/24/2024 - 11:49 The European orphan drug market presents a significant opportunity for biotech firms.













































Let's personalize your content